Dr Manav Pathania
Investigating heterogeneity and microenvironmental interactions in brain tumours and neural stem cells
Departmental affiliation: Department of Oncology
Biography
Manav received a BA in Biology from Grinnell College, in Iowa, and received his PhD in Cell Biology from Yale University, in Connecticut. At Yale he investigated microRNA control of postnatal neural stem cell differentiation and survival in Professor Angelique Bordey’s lab. His first postdoctoral appointment was in Professor Josef Kittler’s lab at UCL in London, where he explored the neurobiological consequences of autism and schizophrenia candidate gene misexpression in vitro and in vivo. Following that, in his second postdoctoral appointment Manav developed in vivo models of paediatric high-grade glioma in Professor Paolo Salomoni’s lab at the UCL Cancer Institute. His lab’s current research focuses on developing newer, more refined, immune-competent models of gliomas and using these for CRISPR screening and single cell analyses. The aim is to use these models to identify mutation or tumour subtype-specific interactions between tumour cells and the stromal and immune compartments, with the further aim to evaluate these as targets for precision therapy development. A second focus is to use these models to reveal mechanisms contributing to the emergence of therapy resistance, a pervasive problem in the treatment of high-grade brain tumours.
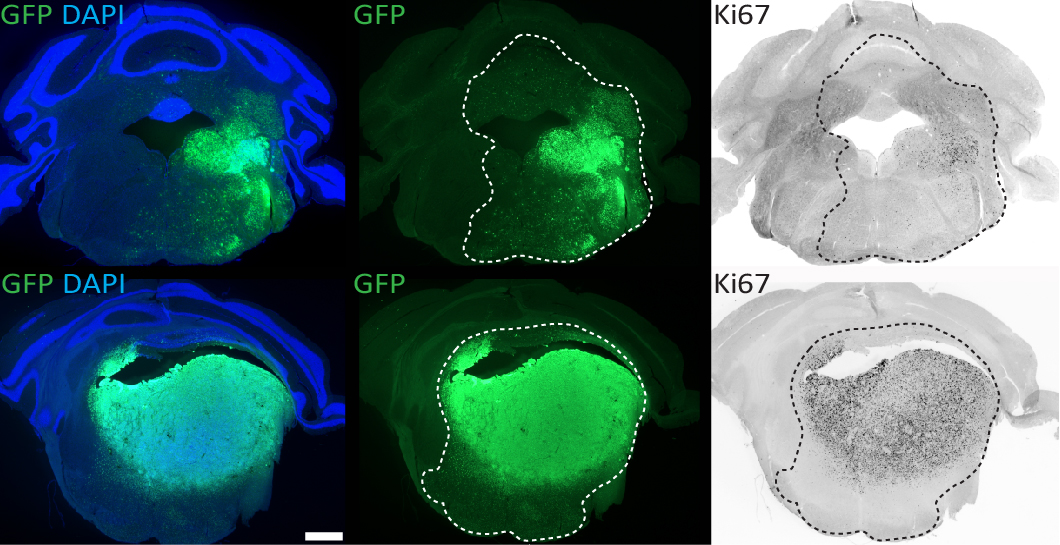
We use in utero or neonatal electroporation to model glioma subtypes driven by different combinations of mutations. Shown here are brainstem tumours driven by different mutations (top vs bottom panels), at 6-8 weeks post-IUE. GFP+ cells that co-label with the proliferative marker Ki67 indicate neoplastic cells actively proliferating within these gliomas. Certain mutations drive tumours that appear more circumscribed/less invasive than others.
Research
We have developed an in vivo brain tumour modelling approach that allows the generation of mouse models more quickly and efficiently than transgenic approaches. This system relies on delivering oncogenic insults into discrete populations of neural stem cells, either in utero or neonatally. We aim to use this approach to test the functional relevance of numerous additional co-occurring mutations that have been found at varying frequencies in paediatric brain cancers. These tumours are some of the most genetically heterogeneous human cancers and are associated with an extremely poor prognosis. Although the co-occurring mutations found within them can be used to stratify tumours into different “subtypes”, the roles that these mutations play in tumour development and relapse are unknown. The research direction we are developing is based on testing mutually exclusive subsets of these mutations for their contribution to tumorigenesis and therapy resistance. This entails developing models of clinically meaningful tumour subtypes and screening them for genetic or microenvironmental dependencies unique to one combination of mutations vs another.
This will allow for a better understanding of the interplay between genetic and phenotypic heterogeneity in different brain tumour types. We are also applying single cell sequencing to these immune-competent models to reveal perturbations in the microenvironment that are unique to the different tumour subtypes, and are carrying out CRISPR screening to identify genetic and epigenetic weaknesses within these tumours. Our hope is that in the longer term we can begin to pave the way to more targeted, precise treatments that can be tailored to the particular combination of mutations unique to each tumour. Key areas of interest also include identifying and functionally validating the precise type of neural stem or progenitor cells that lie at the root of different brain cancer types, and using mouse models to investigate how brain tumours respond and develop resistance to first-line therapies.
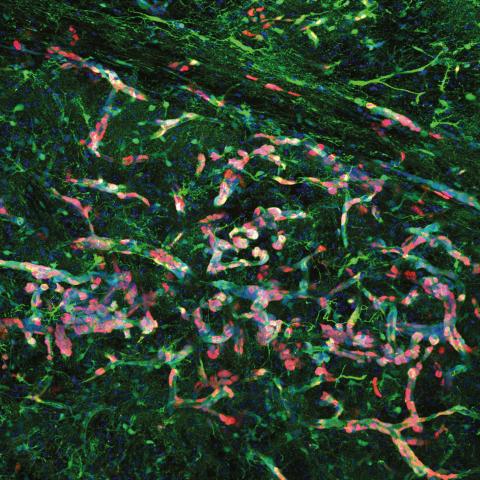
High magnification confocal immunofluorescence of Ki67- and GFP-double positive tumour cells (red and green, respectively) migrating along blood vessels. Nuclei are stained blue.
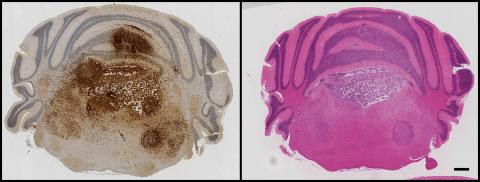
GFP immunohistochemistry and H&E staining in adjacent sections of a mouse model of H3.3K27M-driven diffuse glioma. Tumour cells can be seen proliferating and invading within the hindbrain, spreading into the cerebellum and causing extensive damage to the fourth ventricle.
Additional links