Professor Azim Surani
Specification and programming of the germline for totipotency and development
Email: a.surani@gurdon.cam.ac.uk
Affilation: Gurdon Institute
Biography
Azim Surani was born in Kenya and received PhD in 1975 at Cambridge University under Professor Sir Robert Edwards FRS (Nobel Laureate, 2010). Joined the Babraham Institute in 1979 and discovered Genomic Imprinting in 1984 and subsequently, novel imprinted genes and their functions, with mechanisms through establishment and erasure of DNA methylation. He was elected the Marshall-Walton Professor (1992), and Director of Germline and Epigenomics Research (2013) at Cambridge University.
He has recently established the genetic basis for germ cell specification and epigenetic programming. He was elected a Fellow of the Royal Society (1990) and Fellow of the Academy of Medical Sciences (2001) He was awarded a Royal Medal in 2010 and in 2014 McEwan Award for Innovation, The International Society for Stem Cell Research.
Azim is the Mary Marshall and Arthur Walton Professor of Physiology and Reproduction, and a member of the Physiology, Development and Neuroscience Department, University of Cambridge.
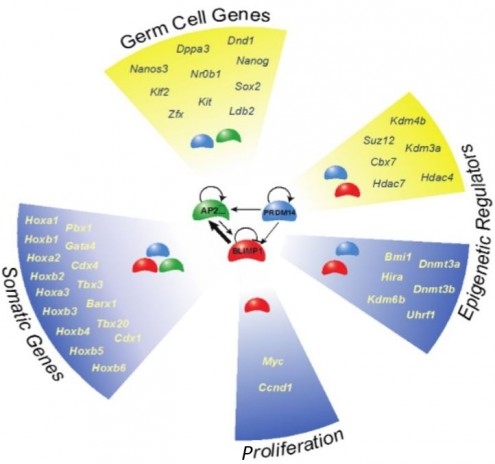
The transcriptional network for mouse primordial germ cell specification. Image: Erna Magnusdottir
Research
Specification of primordial germ cell (PGC) occurs after development of equipotent epiblast cells following their exit from naïve pluripotent state. These epiblast cells can give rise to both somatic and germ cells in vivo and in vitro. Recent studies show that BLIMP1, PRDM14 and AP2g are necessary and sufficient for PGC specification. This mutually interdependent tripartite genetic network is involved in the repression of the somatic program, the initiation of the germ cell program and re-expression of pluripotency genes in early germ cells. The network also initiates sequential, orderly and dynamic epigenetic changes in histone modifications, reactivation of the X chromosome and comprehensive global DNA demethylation and imprints erasure. These epigenetic changes are essential towards imprinting of functional differences between parental genomes and the establishment of the totipotent state, which follows after fertilisation and establishment of the zygote. Whereas a repressive complex maintains unipotency of germ cells, dedifferentiation of unipotent PGCs to pluripotent stem cells in vitro is accompanied by the reversal of the PGC specification process. Early germ cells also exhibit unprecedented genome-wide DNA demethylation and chromatin remodelling, which are essential towards the establishment of totipotency. We are gathering insight into the mechanisms involved in epigenetic programming in germ cells, and continuing to identify the key factors that are crucial at these times.
We are interested in exploiting the knowledge gained from studies on germ cells by creating in vitro models for induced epigenetic reprogramming, and using these models towards attempts at rejuvenation of somatic cells.
CSCI collaborators
Anne Ferguson-Smith (affiliate)