Professor Ludovic Vallier
Stem cells to study liver development and diseases
Laboratory: Berlin Institute of Health at Charité (BIH)
Biography
Ludovic graduated in Molecular biology and Immunology from the University Claude Bernard Lyon I in 1997. In 2001, he earned his PhD at Ecole Normale Superieur of Lyon in the group of Jacques Samarut, under the supervision of Pierre Savatier, studying mechanisms that control the cell cycle in mouse embryonic stem (ES) cells. Following a year in the biotechnology industry, Ludovic joined Professor Pedersen's group at the University of Cambridge Department of Surgery. In 2008 he joined the newly opened Anne McLaren Laboratory for Regenerative Medicine (LRM) as a Principal Investigator and MRC non-clinical senior fellow.
Ludovic was Professor of Regenerative Medicine affiliated to the department of Surgery and director of the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre hIPSCs (human induced pluripotent stem cells) core facility. He was co-deputy director of the Cambridge Stem Cell Institute in 2019, was elected as a Fellow of the Academy of Medical Sciences in 2020 and joined the editorial board of Stem Cell Reports as associated editor. In parallel, he has been involved in the creation of several biotechnology companies including DefiniGEN and BiliTech. Ludovic now serves as Professor of Stem Cells in Regenerative Therapies at the Berlin Institute of Health at Charité (BIH). His group based at the BIH Centre for Regenerative Therapies employs human stem cells to generate cells with a clinical interest for disease modelling and cell-based therapy. Some of his lab remain at the Cambridge Stem Cell Institute.
Funding
European Research Council, CF Trust, Open Target, Milner Institute, Rosetrees Trust, Chan Zuckerberg Initiative and Wellcome Leap.
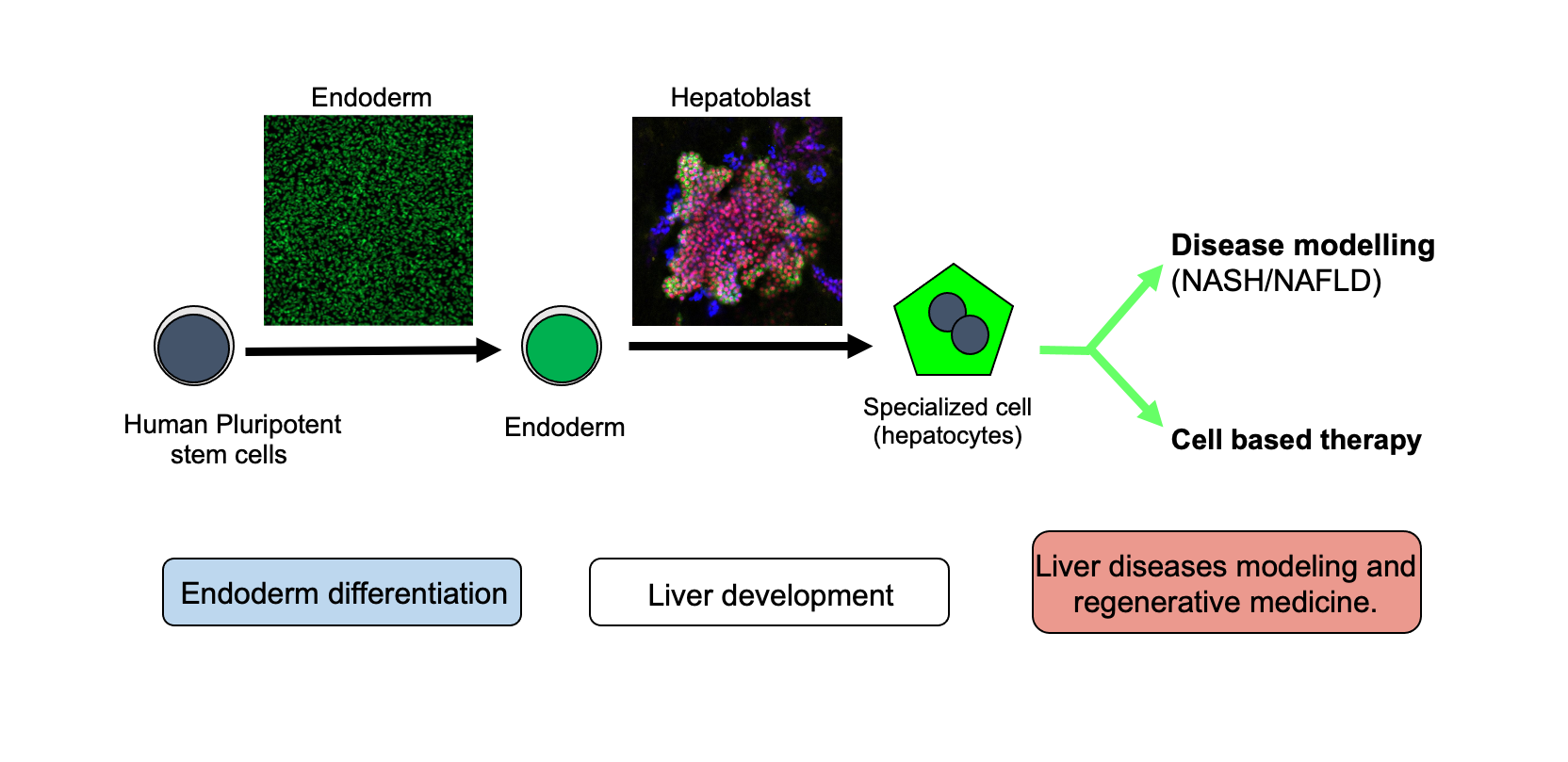
The Vallier Group studies the basic molecular mechanisms controlling cell fate decisions during foetal development and in adult liver. For that, his group utilises human pluripotent stem cells and primary organoids as in vitro model of development. The resulting knowledge is then exploited for generating hepatic cells. The resulting cells are then used for clinical applications including disease modelling and cell-based therapy. (Credit Ludovic Vallier, Katerina Zacharis and Dominika Dziedzicka).
Research
Understanding the mechanisms controlling early cell fate decisions in human development has major importance for regenerative medicine. Indeed, the generation of fully functional cell types from stem cells is only achievable by recapitulating a natural succession of cell fate choice. The first event of differentiation of the embryo proper occurs at the stage of gastrulation with the specification of the three primary germ layers ectoderm, mesoderm and endoderm, from which all the cells of adult tissues are derived. The main objective of the Vallier Group is to define the molecular mechanisms controlling the transition between pluripotency and the endoderm lineage generating hepatic tissue. For that, they use human pluripotent stem cells (hESCs and hIPSCs) and primary organoids as in vitro model of development to study the interplays between transcriptional networks, epigenetic modifications and cell cycle which ultimately orchestrate the organogenesis of the liver. The resulting knowledge allows the development of culture systems to produce not only hepatic cells in vitro but also pancreatic, hepatic, gut and lung cells. These cells are then used to model diseases or for cell-based therapy applications. The group has a specific focus on metabolic disorders affecting the liver including NAFLD/NASH. Their overall objective is to acquire the basic knowledge necessary for generating a diversity of cell types for clinical applications.

Vallier Group photo
Plain English
The objective of our research group is to acquire the basic knowledge necessary to develop new therapies against diseases affecting the liver. For that, we use stem cells to model embryonic development in vitro and to produce liver cells with an interest for cell therapy.
Key Publications
For full list see Google Scholar
-
Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, Upponi SS, Brevini T, Wesley BT, Garcia-Bernardo J, Mahbubani K, Canu G, Gieseck R 3rd, Berntsen NL, Mulcahy VL, Crick K, Fear C, Robinson S, Swift L, Gambardella L, Bargehr J, Ortmann D, Brown SE, Osnato A, Murphy MP, Corbett G, Gelson WTH, Mells GF, Humphreys P, Davies SE, Amin I, Gibbs P, Sinha S, Teichmann SA, Butler AJ, See TC, Melum E, Watson CJE, Saeb-Parsy K*, Vallier L*. (2021) Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021 Feb 19;371(6531):839-846. doi: 10.1126/science.aaz6964. PMID: 33602855. * Joint Authorship.
-
Rimland CA, Tilson SG, Morell CM, Tomaz RA, Lu WY, Adams SE, Georgakopoulos N, Otaizo-Carrasquero F, Myers TG, Ferdinand JR, Gieseck RL 3rd, Sampaziotis F, Tysoe OC, Wesley B, Muraro D, Oniscu GC, Hannan NR, Forbes SJ, Saeb-Parsy K, Wynn TA, Vallier L (2020). Regional differences in human biliary tissues and corresponding in vitro derived organoids. Hepatology. 2021 Jan;73(1):247-267. doi: 10.1002/hep.31252. PMID: 32222998
-
Ortmann D, Brown S, Czechanski A, Aydin S, Muraro D, Huang Y, Tomaz RA, Osnato A, Canu G, Wesley BT, Skelly DA, Stegle O, Choi T, Churchill GA, Baker CL, Rugg-Gunn PJ, Munger SC, Reinholdt LG, Vallier L (2020). Naive Pluripotent Stem Cells Exhibit Phenotypic Variability that Is Driven by Genetic Variation. Cell Stem Cell. 2020 Sep 3;27(3):470-481.e6. doi: 10.1016/j.stem.2020.07.019. PMID: 32795399.PMCID: PMC7487768
-
Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los Mozos IR, Sadée C, Lenaerts AS, Nakanoh S, Grandy R, Farnell E, Ule J, Stunnenberg HG, Mendjan S, Vallier L (2018). The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature. 555(7695):256-259. doi: 10.1038/nature25784. PMID: 29489750/PMCID: PMC5951268
-
Sampaziotis F, Justin AW, Tysoe OC,... Markaki AE, Saeb-Parsy K, Vallier L. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nature Medicine. 2017 Aug;23(8):954-963. PMID:28671689