Submitted by Laura Puhl on Thu, 10/03/2022 - 16:38
A new study was published today (10 March) in Cell Stem Cell, answering a question that’s been sitting unanswered for over thirty years.
The research, led by Professor Simón Méndez-Ferrer’s Lab at Wellcome-MRC Cambridge Stem Cell Institute (CSCI), finally identified Interleukin-6 as a driver of an important switch that links the nervous system and the bone, addressing a long-standing question in the neuroscience field and providing implications for the study of bone health and function.
The nervous system and its signals
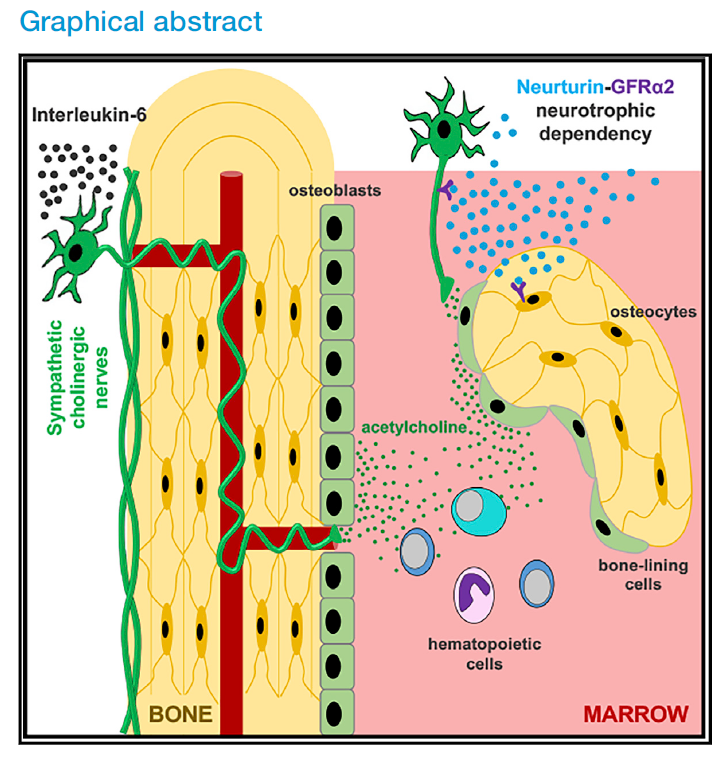
The autonomic nervous system is the regulator responsible for maintaining the processes that keep the body functioning, and includes the sympathetic nervous system (the ‘fight or flight’ response), and the parasympathetic nervous system (the ‘feed and breed, rest and digest’ response). Normally, the sympathetic nerves use noradrenaline to send signals in the body (called noradrenergic), and the parasympathetic nerves use acetylcholine (called cholinergic). However, some sympathetic neurons acquire cholinergic properties through a process known as ‘cholinergic switch’, which happens after birth and allows the sympathetic nerves to communicate using cholinergic signals instead.
While the nervous system’s impact on bone development has been researched previously, showing that the noradrenergic fibres decrease bone mass, the actual role of cholinergic signalling in bone has remained largely unknown. In this research, Méndez-Ferrer and his team, in collaboration with an international multidisciplinary group of researchers including Professor Andrew McCaskie (CSCI) and Professor Pamela Robey (NIH), PhD co-supervisors of the first author of this study (Stephen Gadomski) investigate the cholinergic signalling in order to better understand its impact on bone development in early life, and on bone health later on.
Finding the driver in vivo
Earlier studies showed that Interleukin-6 (IL-6) influences bone formation, but it was primarily studied for its effect on bone degradation, rather than on bone formation. Gadomski, Méndez-Ferrer and his team have now identified IL-6 as the player responsible for flipping a switch in the bone development process. Though this cholinergic switch was first identified in the 1980s, until now the driving factor has never been identified in vivo.
Once this switch occurs, a subset of sympathetic nerve fibres uses acetylcholine to communicate with the bone upon contact, and their message is further amplified through bone-lining osteoprogenitors. This subsequent interaction between the bones and the nervous system is integral to the promotion of bone growth and health during adolescence.
In addition to identifying the driver for this signal, the team also tested whether or not these signals could be instigated through exercise. Using treadmill-running mice and rats, they were able to demonstrate that increased IL-6 during exercise expanded the cholinergic fibres. Because skeletal cholinergic innervation is also present in humans, these results suggest that the achievement of peak bone mass (usually achieved before 30) may be mediated by the sympathetic cholinergic system during adolescence and after physical activity.
In the future, these findings will help scientists better understand the predictors of osteoporosis, and may represent a drug-able target for maintenance of peak bone mass.
Linking bones with blood
This research follows Méndez-Ferrer’s recent paper from Nature Communications, where his team, in collaboration with Professor Bertie Göttgens, demonstrated the sympathetic nervous system’s ability to regulate the activity of blood stem cells in different bone marrow niches. They showed that stress conditions, such as chemotherapy or irradiation, which are routinely used for cancer treatment, increase the signals released by these neurons, which fine tune the control of blood stem cells by their neighbours (stromal cells). As a result, not all blood stem cells become activated (which could cause their exhaustion), but some remain quiescent, thereby retaining their full potential. This mechanism might be important and therapeutically amenable to modulate the recovery of the blood and immune system during regeneration, for instance after blood stem cell transplantation.
Together, these studies illustrate the regenerative function of the sympathetic cholinergic system in the skeletal and haematopoietic systems.