Dr Johannes Bargehr
Immunomodulation in cardiac repair
Email: jb832@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Biography
Johannes Bargehr is a BHF Intermediate Clinical Research Fellow at the University of Cambridge and an honorary cardiology fellow in heart failure at Royal Papworth and Addenbrooke’s hospitals. He graduated from the University of Innsbruck with a degree in medicine and subsequently completed a PhD degree in the Cambridge Stem Cell Institute with Prof Sanjay Sinha. During his PhD, he spent over a year in the Murry lab at the University of Washington to investigate the regenerative potential of human pluripotent stem cell (hPSC)-derived epicardial cells in myocardial remuscularisation. Following his PhD, Johannes continued to work as a post-doctoral fellow in the Sinha lab, while completing his cardiology clinical training in Cambridge.
Johannes’s research uses immunomodulatory strategies to enhance the efficacy of cardiac cell therapy. He also uses hPSC-derived cardiomyocytes in conjunction with immune cells for disease modelling and drug discovery. He is a co-founder of the biotechnology company ABS Biotechnologies GmbH.
Funding
BHF Intermediate Clinical Research Fellowship and BHF CRE
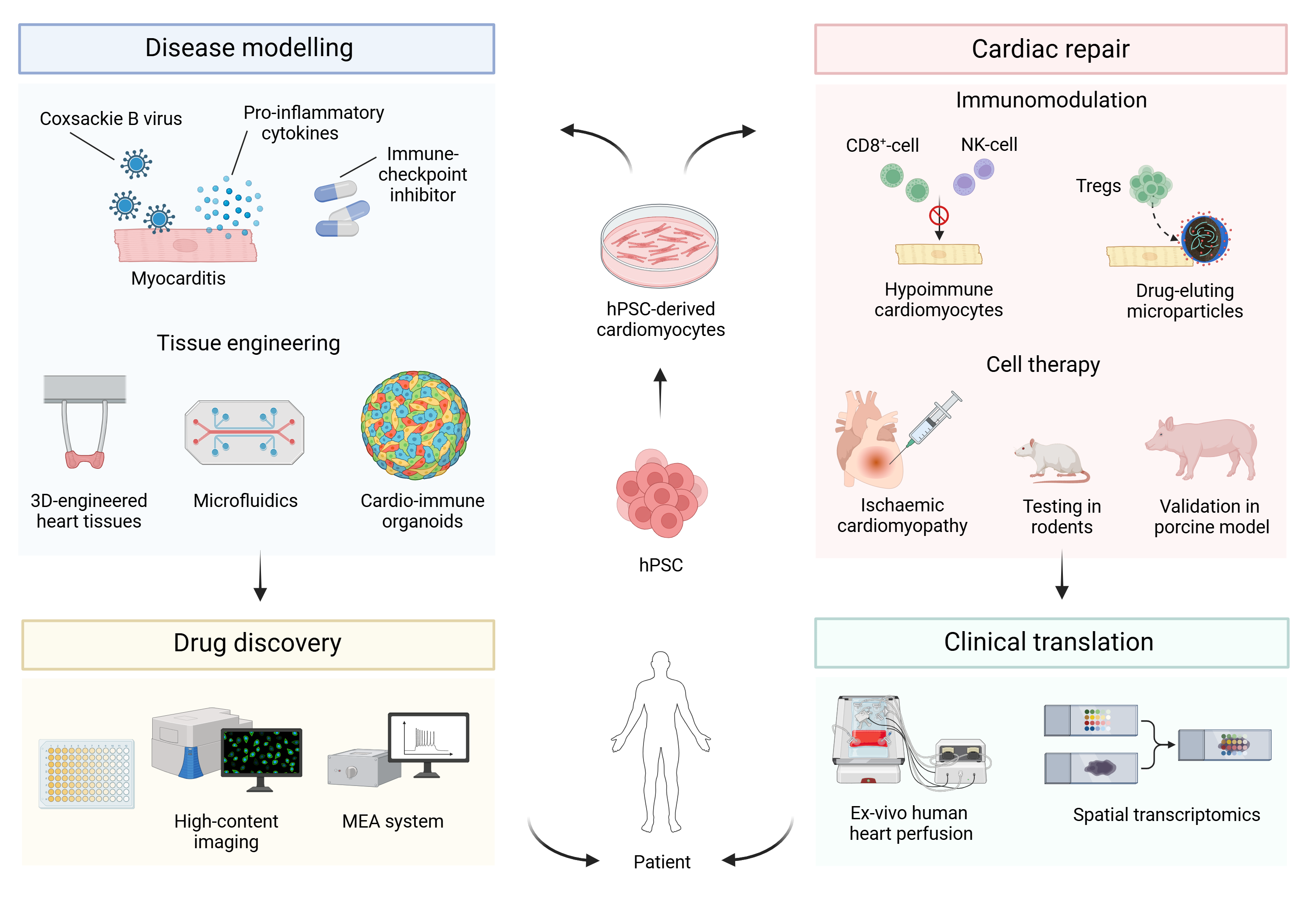
Image: Schematic of research in the Bargehr lab.
Research
The challenge of cardiac repair
Up to 75% of patients do not survive the first 5 years after they have been diagnosed with heart failure. To date there is no scalable clinical therapy that addresses the underlying problem, which is a loss in the contractile working myocardium. It has been shown that hPSC-derived cardiomyocytes can remuscularise infarcted hearts and improve cardiac function in rodents and non-human primates. However, this is a very ineffective process with low cell retention rates and poor engraftment. Furthermore, because hPSC represent an allogeneic cell source, transplantation of hPSC-derived cardiomyocytes will require systemic immunosuppression of patients, which comes with perils on its own, such as renal failure, infection, de novo cardiovascular disease and cancer.
The Bargehr lab uses different immunomodulatory strategies to achieve better grafting efficiency of cardiomyocyte-based cell therapy, including the use of broadly immune-evasive hPSC lines that bypass cytotoxic T-cell, NK-cell, antibody-mediated and phagocytic responses, avoiding the need for systemic immunosuppression. Additionally, we are also testing the application of locally drug-eluting microparticles to avoid systemic administration of immunosuppressants. As myocardial infarction generates a hostile environment for cardiomyocyte engraftment, one line of work assesses how the recruitment of regulatory T-cells can result in a favourable modification of the myocardial niche that will allow for greater cardiomyocyte survival.
Disease modelling and drug discovery
We model cardiac conditions commonly encountered in clinical practice, including myocarditis, using 3D-model systems comprised of hPSC-derived cardiomyocytes and diverse immune cell subsets. We use this disease model to elucidate the underlying mechanism of the disease process resulting in cardiomyocyte death and test if heart muscle can be rescued using different therapeutic compounds. We are also working on a scalable tissue discovery platform that is capable of medium to high throughput compound screening.
Research goals
Our work will inform on key aspects required for successful clinical translation of cardiac repair, including the need for immunosuppression and how to make cardiac cell therapy more efficacious. Cardio-immune models of myocarditis promise to provide mechanistic insights and open up new opportunities for therapeutic intervention.
Plain English
An average heart attack wipes out around 1 billion heart muscle cells, which results in an inability of the heart to pump blood around the body (heart failure). Today there is no effective therapy available to the large number of patients in need of treatment for the loss of heart muscle. Human stem cell-derived heart muscle cells are a promising therapy, but they cannot efficiently replenish the lost heart muscle and they will require patients to take immunosuppressants to avoid a rejection. To overcome these issues we modify our cell lines to make them invisible for the immune system and generate small particles that contain drugs to help heart muscle cells to survive better. We also engineer heart muscle containing immune cells that allows us to model inflammation of the heart in a dish and find new therapies.
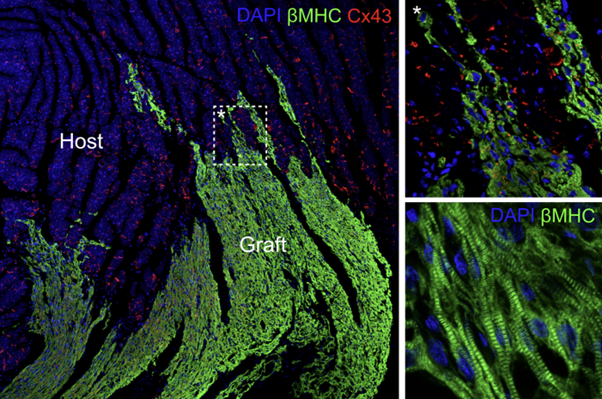
Image: Infarcted rats transplanted with hPSC-derived cardiomyocytes. Human grafts depicted in green