Professor Anna Philpott
Proneural transcription factors
Email: ap113@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Department of Oncology
Biography
Anna Philpott graduated from the University of Cambridge with a BA degree in Natural Sciences in 1988 and a PhD in Molecular Cell Biology in 1991. She held post-doctoral fellowships at Massachusetts General Hospital Cancer Centre in 1992, moving to the Department of Cell Biology at Harvard Medical School in 1993.
She returned to the University of Cambridge in 1998 to a Lectureship in the Department of Oncology, where she is currently Professor of Cancer and Developmental Biology and Pro-Vice-Chancellor for Resources and Operations.
Funding
Cancer Research UK, Wellcome
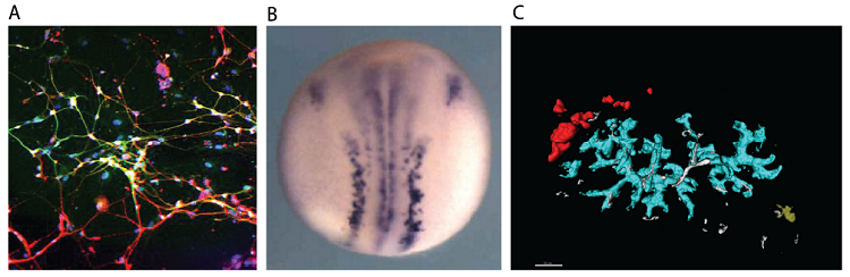
(A) Neurons generated by transcription factor-mediated forward programming. (B) Neurons stained purple in a developing Xenopus frog embryo. (C) Confetti coloured labelling of pancreatic ducts.
Research
The Philpott Lab aims to understand how cells adopt a specific fate and to uncover mechanisms that co-ordinate cell cycling with stem cell maintenance and differentiation during development, homeostasis and disease. Their work has widespread applicability to cellular reprogramming, while we are also looking in detail at perturbation of this co-ordination in the paediatric cancer, neuroblastoma. Recently we have described the mechanism whereby a combination of cyclin-dependent kinase inhibitors and the developmental signal Retinoic Acid can trigger reactivation of a latent differentiation programme in neuroblastoma tumour cells, which may have significant implications for treatments that prevent disease relapse.
The Philpott Lab’s future aims are three-fold:
1. Further characterise the molecular mechanisms that link cell cycling and differentiation
2. Uncover further mechanisms that control the balance between stem-ness/ progenitor maintenance and differentiation in neuroblastoma and other cancers with the aim of developing new therapeutic strategies
3. Probe fundamental mechanisms that determine the fate trajectory and differentiation of different cell types during development focusing on the epigenome and co-factors that control this process at the level of individual cells.

Group Members
Frances Connor, Lidiya Mykhaylechko, Toshiaki Shigeoka, Lydia Parkinson, Laura Woods, William Beckman, Roberta, Azzarelli, Rosalind Drummond
Plain English
Controlling the balance between division of cells and the process of differentiation, whereby cells stop dividing and adopt their specialised function, is critical both in development and in adult tissues that must maintain and repair themselves. Moreover, in many cancers and in particular cancers of children, this balance is disrupted. We are investigating the role of a class of proteins, the proneural factors, in controlling the fate choices cells make as well as their cell division and differentiation, and how their activity is controlled by chemical modification in response to their cellular environment. We are: - Studying how chemical modification of proneural proteins controls cell division versus differentiation in the nervous system, pancreas and gut. - Investigating how proneural proteins work to turn on different genes to either change their fate, promote cell division or arrest it, depending on the environment the cells are in. - Developing treatments for the childhood cancer neuroblastoma by changing the chemical modification of proneural proteins using new drugs. In the longer term, our understanding of the control of this crucial class of proteins, the proneurals, will help us to turn stem cells into useful tissues such as nerve and pancreas, as well as aid us in rational development of new therapies for cancers where proneural protein activity has been disrupted.
Key Publications
-
Palbociclib releases the latent differentiation capacity of neuroblastoma cells. (2023). Ferguson KM, Gillen SL, Chaytor L, Poon E, Marcos D, Gomez RL, Woods LM, Mykhaylechko L, Elfari L, Martins da Costa B, Jamin Y, Carroll JS, Chesler L, Ali FR, Philpott A. Dev Cell. 9;58(19):1967-1982.
-
Super-enhancer associated core regulatory circuits mediate susceptibility to retinoic acid in neuroblastoma cells. Gomez RL, Woods LM, Ramachandran R, Abou Tayoun AN, Philpott A, Ali FR. Frontiers Cell Dev Biol. 2022 10:943924.
-
Tumoral heterogeneity in neuroblastoma. Gomez RL, Ibragimova S, Ramachandran R, Philpott A, Ali FR. Biochim Biophys Acta Rev Cancer. 2022 Nov;1877(6):188805. doi: 10.1016/j.bbcan.2022.188805. Epub 2022 Sep 24.
-
The proneural transcription factor ASCL1 regulates cell proliferation and primes for differentiation in neuroblastoma. Parkinson LM, Gillen SL, Woods LM, Chaytor L, Marcos D, Ali FR, Carroll JS, Philpott A. Frontiers Cell Dev Biol. 2022 Oct 3;10:942579. doi: 10.3389/fcell.2022.942579. eCollection 2022.
-
p57Kip2 imposes the reserve stem cell state of gastric chief cells. (2022). Ji-H. Lee, S .Kim S. Han J. Min B.Caldwell, A. Bamford, A. Rocha, H. Lee, J. Fink, S.Pilat-Carotta, J.Kim, M. Josserand, J.Park, S.Lee, R. Szep-Bakonyi , Y.Ahn, Y. Seok Ju, A. Philpott, B. D. Simons, D. E. Stange, E. Choi5, BK. Koo, and Jong Kyoung Kim. Cell Stem Cell 29, 826-839.
-
Elevated ASCL1 activity creates de novo regulatory elements associated with neuronal differentiation. (2022). Woods LM, Ali FR, Gomez R, Chernukhin I, Marcos D, Parkinson LM, Tayoun ANA, Carroll JS, Philpott A. BMC Genomics. 23(1):255.
-
ASCL1 phosphorylation and ID2 upregulation are roadblocks to glioblastoma stem cell differentiation. (2022). Azzarelli R, McNally A, Dell'Amico C, Onorati M, Simons B, Philpott A.Sci Rep.;12(1):2341.
-
Tracing oncogene-driven remodelling of the intestinal stem cell niche. M.K.Yum , S. Han , J. Fink, S.S. Wu, C. Dabrowska, T. Trendafilova, R. Mustata, L. Chatzeli, R. Azzarelli, I. Pshenichnaya, E.Lee, F. England, J.K. Kim, D.E. Stange, A. Philpott, J.H. Lee, B.K.Koo B.D Simons. Nature. 2021 Jun;594(7863):442-447. doi: 10.1038/s41586-021-03605-0. Epub 2021 Jun 2.PMID: 34079126
-
Three-dimensional model of glioblastoma by co-culturing tumor stem cells with human brain organoids. 2021.R. Azzarelli, M. Ori , Philpott A, B. D.Simons .Biol Open. 2021 Feb 22;10(2):bio056416. doi: 10.1242/bio.056416.
-
Dephosphorylation of the Proneural Transcription Factor ASCL1 Re-Engages a Latent Post-Mitotic Differentiation Program in Neuroblastoma. F.R. Ali, D. Marcos, I. Chernukhin , L.M. Woods, L.M Parkinson, L.A. Wylie, T.D. Papkovskaia,, J.D. Davies, J.S. Carroll, Philpott A. Mol Cancer Res. 2020 Dec;18(12):1759-1766. doi: 10.1158/1541-7786.MCR-20-0693. Epub 2020 Oct 12.


