Dr Florian Merkle
Human stem cell models of obesity and neurological disease
Email: fm436@medschl.cam.ac.uk
Departmental Affiliation: Wellcome-MRC Institute of Metabolic Sciences
Research
The Merkle laboratory aims to uncover the mechanistic basis of human neurological diseases using human pluripotent stem cell (hPSC)-derived culture systems in order to facilitate the development of effective treatments. We use a variety of techniques including CRISPR/Cas9-based genome engineering, single-cell transcriptomics, quantitative proteomics, high content imaging, calcium imaging, and organoid and other co-culture systems. These studies are funded by the Royal Society and Wellcome Trust, the New York Stem Cell Foundation and the Chan Zuckerberg Initiative. Our research focuses on three main areas:
1) Models of obesity and neurodegeneration
Obesity and neurodegeneration lead to millions of premature deaths each year and lack broadly effective treatments. Obesity is largely caused by the abnormal function of cell populations in the hypothalamus that regulate appetite. We generate human hypothalamic neurons from hPSCs to study how they respond to nutrients and hormones (e.g. leptin) and how disease-associated mutations alter their function. Since human hypothalamic neurons can be produced in large numbers, are functionally responsive, have a human genome that can be readily edited, and are in culture environment that can be readily controlled, there is an unprecedented opportunity to study the genetic and environmental factors underlying obesity. In addition, we are fascinated by the fact that mid-life obesity is a risk factor for dementia later in life, and caloric restriction, exercise, and certain anti-obesity drugs are neuroprotective, suggesting that there are shared mechanisms between obesity and neurodegeneration. We are exploring these interactions in a new line of investigation for our group, using a combination of in vitro co-culture models and in vivo models. These studies may help bridge the mechanistic gulf between human genetic data and organismic phenotypes, revealing new therapeutic targets.
2) Therapeutic translation
The functionality and scalability of hPSC-derived cellular systems makes them an attractive system for identifying and testing new therapeutic compounds. In particular, we plan to extend our observations from genetic and phenotypic studies of hPSC-derived neurons to test for factors that can reduce disease phenotypes or promote the production of appetite-suppressing neuropeptides such as beta-MSH (Kirwan et al., Mol. Met., 2018) using single-cell transcriptomics and high-content imaging. We then plan to advance promising compounds into in vivo models to test their ability to reduce obesity, or delay the onset or slow the progression of neurodegeneration.
3) Genetic stability in human stem cells
HPSCs are widely used to study development or model disease in vitro, and to generate cellular products for human transplantation to restore function lost in disease. However, hPSCs accumulate mutations in culture that could compromise both the reproducibility of in vitro studies and the safety of regenerative medicine approaches. For example, we recently showed that hPSCs recurrently acquire cancer-associated mutations in the tumour suppressor TP53 (p53) that promote growth in culture and would increase the risk of cancer formation from transplanted cells (Merkle et al., Nature, 2017). It is therefore critical to understand which mutations are likely deleterious, and to reduce the rate at these mutations accumulate in culture. In collaboration with the UK Regenerative Medicine Platform we are carrying out whole genome and whole exome sequencing to understand the effects of gene editing on hPSC genetic architecture and developing novel methods to identify culture conditions that reduce selective pressures in order to reduce the rate at which mutations accumulate.
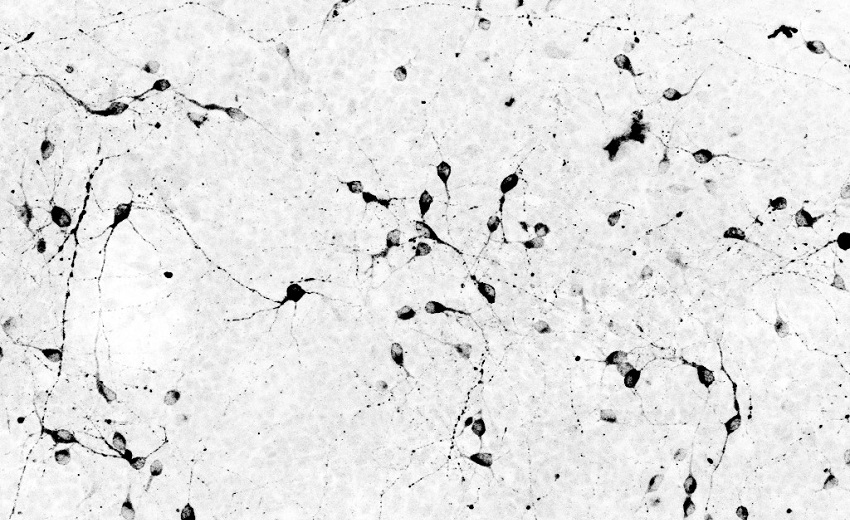
Inverted photomicrograph of human hypothalamic neurons differentiated from pluripotent stem cells, immunostained for pro-opiomelanocortin (POMC). POMC neurons play a pivotal role in inhibiting feeding, and their dysfunction can cause obesity. The Merkle laboratory uses in vitro cellular models of human POMC neurons to unravel the genetic and environmental factors that contribute to human obesity. Image credit: Peter Kirwan
Plain English
I am interested in how human genetic variation contributes to disease, in particular obesity and sleep disorders. These diseases are due to the loss or dysfunction of particular types of neurons (brain cells) found in a region of the brain known as the hypothalamus. Hypothalamic neurons can be generated in a culture dish from human stem cells. Our laboratory uses a combination of cutting-edge approaches to determine how disease associated genes lead to disease states at the level of cells (in human stem cell-derived neurons) and organisms (in gene edited mice).
Vacancies
The Merkle Lab is always happy to hear from outstanding graduate students or postdoctoral candidates. Contact fm436@medschl.cam.ac.uk for more.
ORCID: 0000-0002-8513-2998
Additional links
Cambridge Neuroscience Network
CSCI collaborators


