Dr Maria Alcolea
Epithelial cell fate and plasticity
Email: mpa28@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Oncology
Biography
In 2007 Maria received her PhD in Biochemistry at the University of the Balearic Islands, Spain. She then moved to Barts Cancer Institute - Queen Mary University of London to start her Postdoctoral training with Dr. P.R. Cutillas, where she used phosphoproteomic approaches to study cancer drug resistance. In 2009 she joined Prof Phil Jones laboratory at the Hutchison/MRC cancer unit where she was awarded a Marie Curie Intra-European Fellowship (FP7). There she spent a total of 6 years studying epithelial stem cell behaviour using genetic lineage tracing approaches and methods from statistical physics.
In 2015, Maria was awarded a Wellcome/The Royal Society Sir Henry Dale Fellowship to establish her own laboratory to study epithelial stem cell plasticity in response to injury and tumour development.
Maria is currently a Principal Investigator at the Wellcome-MRC Cambridge Stem Cell Institute and affiliate to the Oncology Department, University of Cambridge.
Funding
Wellcome, Royal Society, Isaac Newton Trust, Medical Research Council, Worldwide Cancer Research, The Leverhulme Trust
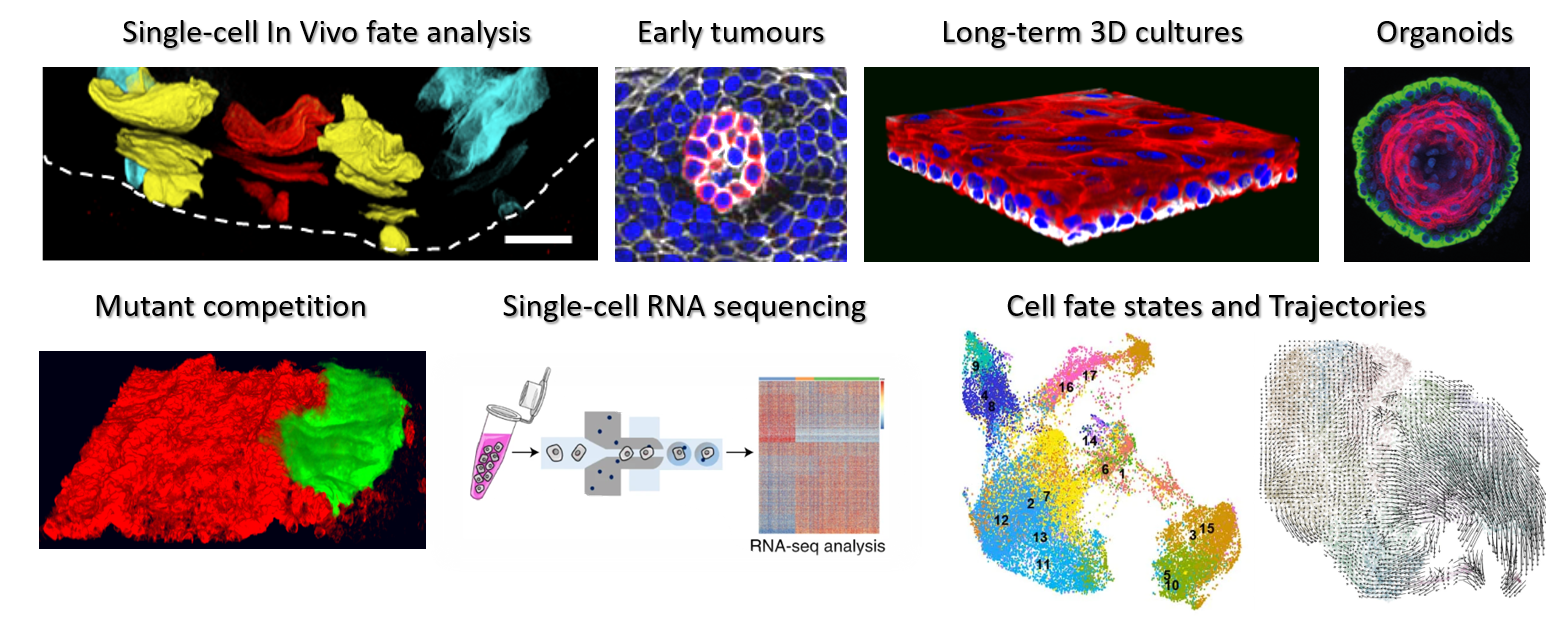
The study of stem cell dynamics by combining fate mapping, live-imaging, and single-cell molecular profiling of in vivo and 3D in vitro epithelial systems with the aim to improve regeneration, prevent cancer formation and ageing.
Research
Maria's research interests have been focused on studying the behaviour of progenitor cells in the mouse oesophagus as a model to unveil the basic rules underlying squamous epithelial cell fate. Her work in the field has revealed how this tissue is maintained under homeostatic conditions, and how these rules switch upon injury.
More recently she has been able to identify how progenitor cells alter and adapt their behaviour in response to preneoplastic mutations, reflecting their remarkable cellular plasticity. Investigating the cellular and molecular mechanisms governing this dynamic behaviour and the potential implications for early cancer development will constitute the basis of my research programme.
To answer these questions, she plans to make use of a combination of in vivo lineage tracing techniques, transcriptional network analysis, as well as 3D organoid and explant culture systems.
Alcolea Group photo
Plain English
Epithelial cells have the essential role of protecting us from external aggressions. However, this critical barrier must be able to adapt in order to face changes during developmental tissue formation and wound healing. A cut in our skin activates a number of cellular responses ensuring that the breach is fixed in few days, recovering the protective barrier. However, given that development and wound healing require the production of a significant amount of new tissue in a relatively short time, it is not surprising that cancer cells mimic these processes to rapidly produce a tumour mass. The difference being that tissue formation and wound repair are very controlled processes, while cancer is not. My proposed research aims to investigate these adaptive cellular responses and the molecular mechanisms behind them in order to understand epithelial tissue behaviour, and how this can go awry during cancer development.
Key publications
- Herms A*, Fernandez-Antoran D*, Alcolea MP*, Kalogeropoulou A, Banerjee U, Piedrafita G, Abby E, Valverde-Lopez JA, Ferreira IS, Dentro SC, Ong SH, Colom B, Murai K, King C, Mahbubani K, Saeb-Parsy K, Lowe AR, Gerstung M, Jones PH. Epithelioids: Self-sustaining 3D epithelial cultures to study long-term processes. PREPRINT, bioRxiv. 2023, 522589
- Colom B, Herms A, Hall MWJ, Dentro SC, King C, Sood RK, Alcolea MP, Piedrafita G, Fernandez-Antoran D, Ong SH, Fowler JC, Mahbubani KT, Saeb-Parsy K, Gerstung M, Hall BA, Jones PH. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature. 2021 Oct;598(7881):510-514.
- Bejar MT*, Jimenez-Gomez P*, Moutsopoulos I, Colom B, Han S, Calero-Nieto FJ, Göttgens B, Mohorianu I, Simons BD, Alcolea MP. Defining the transcriptional signature of esophageal-to-skin lineage conversion. PREPRINT, bioRxiv. 2021, 431899
- McGinn J, Hallou A, Han S, Krizic K, Ulyanchenko S, Iglesias-Bartolome R, England FJ, Verstreken C, Chalut KJ, Jensen KB, Simons BD, Alcolea MP. A biomechanical switch regulates the transition towards homeostasis in esophageal epithelium. Nat Cell Biol. 2021 May;23(5):511-525.
- Colom B, Alcolea MP, Piedrafita G, et al. Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat Genet. 2020 Jun;52(6):604-614
- Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, Jones PH. Differentiation imbalance in single Oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol. 2014 Jun;16(6):615-22
- Doupé DP*, Alcolea MP*, Roshan A, Zhang G, Klein AM, Simons BD, Jones PH. A Single Progenitor Population Switches Behavior to Mainta in and Repair Esophageal Epithelium. Science. 2012 Aug 31;337(6098):1091-3.