Professor Cédric Ghevaert
In vitro production of platelets for transfusion
Email: cg348@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Haematology
Biography
Cédric Ghevaert graduated from the medical school of the University Libre de Bruxelles in 1997 and subsequently became a fellow of the Royal College of Physicians, London (2000). He specialised in Haematology and became a fellow of the Royal College of Pathologists in 2005. He obtained his PhD in 2008 studying novel antibodies for the treatment of bleeding in neonates in Cambridge. He obtained a personal Intermediate Clinical Fellowship from the British Heart Foundation whilst working in Prof Steve Watson's group at the university of Birmingham in 2009.
He took up the post of Senior Lecturer in Transfusion Medicine at the University of Cambridge in 2010, a post funded by the NHS Blood and Transplant and in 2020 was promoted to Reader in Transfusion Medicine. In addition to his academic post, Professor Ghevaert also works as a Consultant Haematologist for the NHSBT.
Funding
Wellcome Trust, MRC, NHS Blood and Transplant, EU, UK Regenerative Medicine Platform.
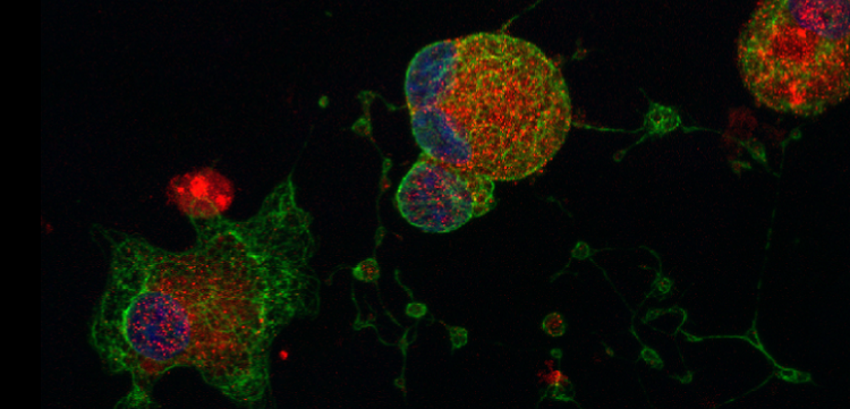
Megakaryocytes were produced from human pluripotent stem cells through over expression of 3 key transcription factors TAL1, GATA1 and FLI1. These cells are capable of producing platelets that contain the granules (in red=P-selectin; green=alpha-tubulin; blue=nuclei) necessary to perform their clotting function after transfusion.
Research
The main focus of the Ghevaert group’s research is the production of blood cells for human use, namely red cells and platelets. They have developed a particular expertise in the production of these cell types from human pluripotent stem cells using methodologies that are compatible with the production of clinical grade products within the constraints of affordable manufacturing processes. To this end they are combining cellular programming through knowledge and manipulation of transcription factor networks and the creation of 3D biocompatible niches and bioreactors.
As a consultant haematologist for the NHS Blood and Transplant (a partner organisation of the University of Cambridge), Cedric has expertise recognised world-wide in carrying out first-in-man studies of manufactured blood cell survival and recovery in human volunteers. The RESTORE trial carried out in partnership with NHSBT, the Clinical Research Facility (Cambridge University NHS Foundation Trust) and Guy’s Hospital Radiopharmacy looks at survival of manufactured red cells post-transfusion is due to complete in 2024.

Ghevaert Group photo
Plain English
We are currently entirely reliant on blood donors for the red cells and platelets transfusion we administer to patients who are very anaemic or at high risk of bleeding. Producing these blood cells in the laboratory would take the pressure of the supply chain and would make finding compatible blood for patients with rare blood groups easier. We are developing novel methods to produce red cells and platelets from human stem cells by using key "identity switches" and programming the stem cells to become blood cells. This method generates highly pure cell harvest with large quantities of blood cells to the point that we are now setting up clinical trials to assess these cells in human volunteers.
| Watch the group's thoughts on blood transfusions of the future: | |
| Watch an interview with the Ghevaert Group: | |
| Watch an introduction to the Horizon 2020 SilkFusion project: | |
Key Publications
-
Zhao X, Alibhai D, ... Ghevaert C, Poole AW. Highly efficient platelet generation in lung vasculature reproduced by microfluidics. Nat Commun. 2023 Jul 7;14(1):4026. doi: 10.1038/s41467-023-39598-9. PMID: 37419900
-
Bennett C, Lawrence M, ... Ghevaert C. CRLF3 plays a key role in the final stage of platelet genesis and is a potential therapeutic target for thrombocythemia. Blood. 2022 Apr 7;139(14):2227-2239. doi: 10.1182/blood.2021013113. PMID: 35051265
-
Evans AL, Dalby A, ... Ghevaert C. Transfer to the clinic: refining forward programming of hPSCs to megakaryocytes for platelet production in bioreactors. Blood Adv. 2021 Apr 13;5(7):1977-1990. doi: 0.1182/bloodadvances.2020003236. PMID: 33843988
- Waller AK, Lantos L, Sammut A, Salgin B, McKinney H, Foster HR, Kriek N, Gibbins JM, Stanworth SJ, Garner SF, Venkatesh V, Curley A, Belteki G and Ghevaert C. Flow cytometry for near-patient testing in premature neonates reveals variation in platelet function: a novel approach to guide platelet transfusion. Pediatr Res. 2019;85:874-884.
- Shepherd JH, Howard D, Waller AK, Foster HR, Mueller A, Moreau T, Evans AL, Arumugam M, Bouet Chalon G, Vriend E, Davidenko N, Ghevaert C*, Best SM* and Cameron RE*. Structurally graduated collagen scaffolds applied to the ex vivo generation of platelets from human pluripotent stem cell-derived megakaryocytes: Enhancing production and purity. Biomaterials. 2018;182:135-144.
- Dalby A, Ballester-Beltran J, Lincetto C, Mueller A, Foad N, Evans A, Baye J, Turro E, Moreau T, Tijssen MR and Ghevaert C. Transcription Factor Levels after Forward Programming of Human Pluripotent Stem Cells with GATA1, FLI1, and TAL1 Determine Megakaryocyte versus Erythroid Cell Fate Decision. Stem Cell Reports. 2018;11:1462- 1478.
- Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, Howard D, Colzani M, Arumugam M, Wu WH, Dalby A, Lampela R, Bouet G, Hobbs CM, Pask DC, Payne H, Ponomaryov T, Brill A, Soranzo N, Ouwehand WH, Pedersen RA and Ghevaert C. Large- scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun. 2016;7:11208.
- Ghevaert C, Herbert N, Hawkins L, Grehan N, Cookson P, Garner SF, Crisp-Hihn A, Lloyd- Evans P, Evans A, Balan K, Ouwehand WH, Armour KL, Clark MR and Williamson LM. Recombinant HPA-1a antibody therapy for treatment of fetomaternal alloimmune thrombocytopenia: proof of principle in human volunteers. Blood. 2013;122:313-20.