Professor Simón Méndez-Ferrer
Blood stem cell niches
Email: sm2116@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Haematology
Biography
Professor Méndez-Ferrer gained his PhD in 2004 from The Department of Medical Physiology at the University of Seville in Spain. His Thesis characterized properties of the carotid body which is of potential interest for neuroregenerative strategies which in turn is based on the biological delivery of catecholamines and neurotrophic factors. From 2006-9, Dr Mendez- Ferrer worked as a post-doc at Mount Sinai School of Medicine in New York, USA and discovered that hematopoietic stem cell traffic is regulated by circadian oscillations.
His subsequent work as an Assistant Professor at the Icahn School of Medicine at Mount Sinai (New York) and at the Spanish National Centre for Cardiovascular Research (CNIC, Madrid) (2009-10) identified self-renewing mesenchymal stem cells that have a crucial role in the hematopoietic stem cell niche. Dr Mendez-Ferrer moved to Cambridge in 2015 as Reader and later on Professor at the Department of Hematology (University of Cambridge), Group Leader at the Wellcome-MRC Cambridge Stem Cell Institute and PI at NHS Blood and Transplant. He now focuses on investigating novel physiological mechanisms that regulate the stem cell niche and contribute to pathogenesis.
Blood stem cells reside in specialised niches, which allows them to self-renew, proliferate, differentiate and migrate according to the organism's requirements. His research has revealed multisystem regulatory mechanisms by which the haematopoietic stem cell niche fulfils these complex functions and how the deregulation of these mechanisms contributes to haematological disorders. His research has discovered a connection between the bone marrow, the brain and other systemic signals, which regulate the behaviour of blood stem cells.
Funding
ERC, NHS Blood and Transplant, Cancer Research UK, MRC
External links
https://www.haem.cam.ac.uk/staff/senior-staff/dr-simon-mendez-ferrer/
https://crukcambridgecentre.org.uk/users/simon-mendez-ferrer
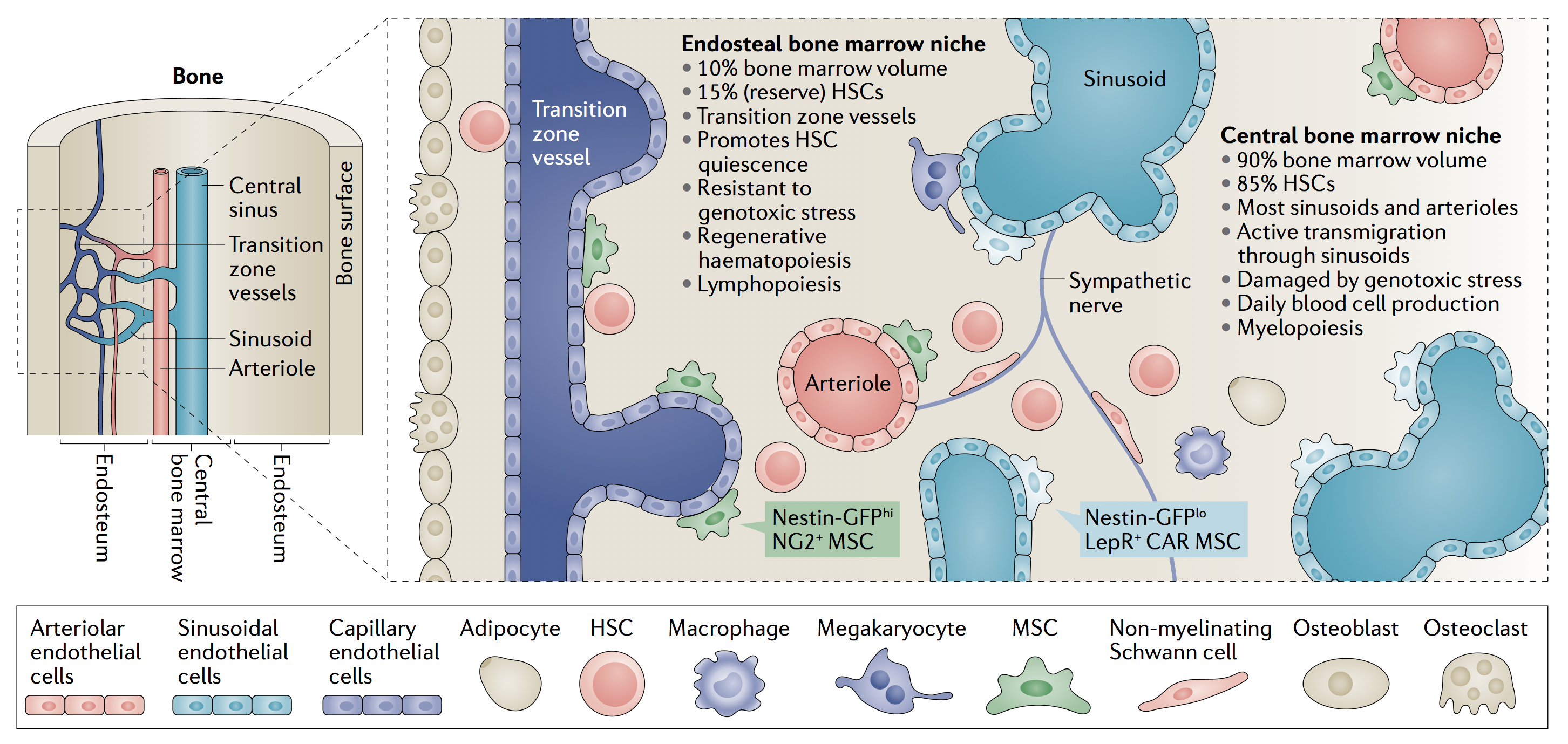
Main features of anatomically-defined haematopoietic stem cell niches in the mouse bone marrow. Schematic summarising the key cell types and functional features of the central and endosteal bone marrow (BM) niches of haematopoietic stem cells (HSCs). Mesenchymal stem cells (MSCs) give rise to bone-forming cells (osteoblasts) and fat cells (adipocytes), whereas bone-resorbing cells (osteoclasts) share a monocytic origin with macrophages. Nes-GFPhi NG2+ MSCs are associated with endosteal transition zone vessels and arterioles located throughout the BM, whereas Nes-GFPlo LEPR+ CAR MSCs are associated with sinusoids in the central BM. Sympathetic nerve fibres regulate the migration of HSCs through the sinusoids. Different MSC subpopulations, endothelial cells, non-myelinating Schwann cells and megakaryocytes might contribute to regulate the balance between HSC proliferation and quiescence during daily or regenerative haematopoiesis. Changes in specialized BM niches might directly affect myeloid versus lymphoid output, and the imbalanced production of mature haematopoietic cells at specific niches might in turn remodel the local microenvironment for these cells. During ageing, the landscape of the mouse HSC niche changes substantially. Specifically, the central niche expands with morphological and functional changes including an increase in noradrenergic fibres, capillaries with Nes-GFP+ MSCs, HSC proliferation and myelopoiesis. In contrast, the endosteal niche and its associated components and functions are reduced with concomitant decreases in transition zone vessels and arterioles, HSC quiescence and self-renewal, resistance to genotoxic stress, regenerative haematopoiesis and lymphopoiesis (Credit Mendez-Ferrer S et al. Nat Rev Cancer 2020;20(5):285-98).
Research
The Méndez-Ferrer laboratory research focuses on the regulation of the haematopoietic stem-cell niche in health and disease. Blood stem cells reside in specialised niches which allows them to self-renew, proliferate, differentiate and migrate according to the organism's requirements. The group studies multisystem regulatory mechanisms by which the haematopoietic stem cell niche fulfils these complex functions and how the deregulation of these mechanisms contributes to haematological disorders. A connection was established between the bone marrow, the brain and other systemic signals, which regulate the behaviour of haematopoietic stem cells (HSCs). Additionally, the group has demonstrated that mesenchymal stem cells (MSCs) are very important in supporting and regulating HSCs. This is also the case for malignant HSCs that can cause myeloproliferative disorders, such as myeloproliferative neoplasms (MPNs) and acute myeloid leukaemia (AML). The group found that malignant HSCs damage their niche to drive disease progression, prompting for strategies targeting the niche, which are under clinical evaluation. Current group’s efforts focus on the neuro-endocrine regulation of the HSC niches to improve HSC transplantation procedures and the treatment of myeloproliferative neoplasias.
Méndez-Ferrer group photo
Plain English
Our research focuses on the regulation of the environment (‘niche’) in which the blood stem cells reside both in health and disease. Blood stem cells are located in specialised niches which allow them to function according to the organism's requirements. We study the mechanisms of how the stem cell niche fulfils these complex functions. Through this, we aim to understand how the disruption of these mechanisms contributes to blood disorders.
Key Publications
- Grockowiak E, Korn C, Rak J, Hallou A, Lysenko V, Panvini FM, Fieding C, Williams M, Khatib-Massalha E, Fang Z, García-García A, Korshed RA, González-Antón S, Li J, Baxter EJ, Kusumbe A, Wilkins BS, Green A, Simons BD, Harrison CN, Green AR, Lo Celso C, Theoccarides APA and Méndez-Ferrer S. Different niches for mutant stem cells affect pathogenesis and therapy response in myeloproliferative neoplasms. Nat Cancer (In Review), available at Research Square [https://doi.org/10.21203/rs.3.rs-1875241/v1].
- Gadomski S, Fielding C, García-García A, Korn C, Kapeni C, Ashraf S, Villadiego J, del Toro R, Domingues O, Skepper JN, Michel T, Zimmer J, Sendtner R, Dillon S, Poole K, Holdsworth G, Sendtner M, Toledo-Aral JJ, De Bari C, McCaskie AW, Robey RG and Méndez-Ferrer S. A cholinergic neuroskeletal interface promotes bone formation during postnatal growth and exercise. Cell Stem Cell 29, 2022; doi: 10.1016/j.stem.2022.02.008. PMID: 35276096.
- Fielding C, García-García A, Korn C, Gadomski S, Fang Z, Reguera JL, Pérez-Simón JA, Göttgens B and Méndez-Ferrer S. Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis. Nat Commun. 2022 Jan 27;13(1):543. doi: 10.1038/s41467-022-28175-1. PMID: 35087060.
- Méndez-Ferrer S§, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN and Frenette PS§. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829-834 (§corresponding authors). PMID: 20703299
- Forte D, García-Fernández M, Sánchez-Aguilera A, Stavropoulou V, Fielding C, Martín-Pérez D, López JA, Costa ASH, Tronci L, Nikitopoulou E, Barber M, Gallipoli P, Marando L, Fernández de Castillejo CL, Tzankov A, Dietmann S, Cavo M, Catani L, Curti A, Vázquez J, Frezza C, Huntly BJ, Schwaller J and Méndez-Ferrer S. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metabolism. 2020 Sep 16:S1550-4131(20)30479-4. doi:10.1016/j.cmet.2020.09.001 PMID: 32966766
- Ho YH, Del Toro R, Rivera-Torres J, Rak J, Korn C, García-García A, Macías D, González-Gómez C, Del Monte A, Wittner M, Waller AK, Foster HR, López-Otín C, Johnson RS, Nerlov C, Ghevaert C, Vainchenker W, Louache F, Andrés V and Méndez-Ferrer S. Remodelling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 2019 Sep 5;25(3):407-418.e6. doi:10.1016/j.stem.2019.06.007. PMID: 31303548
- García-García A, Korn C, García-Fernández M, Domingues O, Villadiego J, Martín-Pérez D, Isern J, Bejarano-García JA, Zimmer J, Pérez-Simón JA, Toledo-Aral JJ, Michel T, Airaksinen MS, Méndez-Ferrer S. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood. 2019 Jan 17;133(3):224-236. doi: 10.1182/blood-2018-08-867648. PMID: 30361261
- Sánchez-Aguilera A, Arranz L, Martín-Pérez D, García-García A, Stavropoulou V, Kubovcakova L, Isern J, Martín-Salamanca S, Langa X, Skoda RC, Schwaller J, Méndez-Ferrer S. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell Stem Cell 15: 791-780, 2014. PMID:25479752
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng Y-S, Lai D-M, Schwaller J, Skoda RC, Méndez-Ferrer S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512: 78-81, 2014. PMID:25043017