Dr Fotios Sampaziotis
Hepatobiliary disorders
Email: fs347@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Medicine
Biography
Fotios Sampaziotis is a UK Research and Innovation (UKRI) Future Leaders Fellow at the University of Cambridge and an honorary consultant hepatologist in Addenbrooke’s hospital. He obtained his medical degree from the University of Athens and completed his hepatology clinical training in Cambridge. In parallel, he secured an MRC Clinical Research Training Fellowship towards a PhD degree in the Cambridge Stem Cell Institute and continued his post-doctoral research as an NIHR Clinical Lectureship in Hepatology with Prof Ludovic Vallier in Cambridge.
Fotios’ research combines cholangiocyte biology, bioengineering and regenerative medicine to develop new therapies for biliary disease. He is a founding member of the European Association for the Study of Liver (EASL) Regenerative Hepatology Consortium and a co-founder of the biotechnology company Bilitech LTD.
Funding
Rosetrees Trust and UKRI
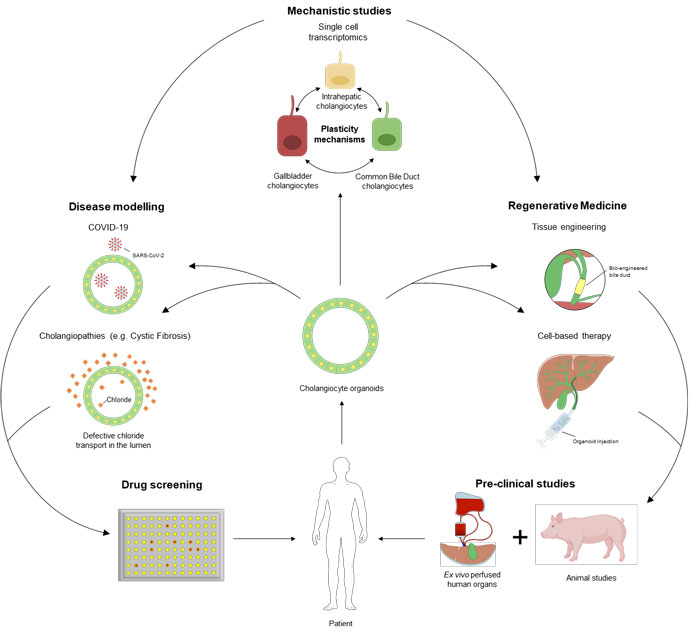
Legend: Schematic overview of research in the Sampaziotis lab
Research
Biliary disorders (cholangiopathies) are a leading cause for transplantation with minimal therapeutic alternatives. We use patient tissue, single cell transcriptomics and bile duct cell (cholangiocyte) organoids to better understand how bile ducts regenerate, why these mechanisms fail in disease, and whether we can promote endogenous regeneration as a therapeutic strategy to repair duct damage.
We discovered that duct regeneration is closely related to cholangiocyte plasticity. We mapped cholangiocyte populations in the biliary tree using single cell transcriptomics and found that the biliary epithelium is made up of different cell types. However, following damage any cholangiocyte can change their identity to replenish the cell types which have been destroyed and this process is guided by environmental stimuli, such as bile acids. We are trying to understand why this mechanism fails in disease and identify therapeutics modifying the cells’ microenvironment to promote endogenous regeneration. Furthermore, we aim to explore whether this ‘plastic transformation’ represents a universal epithelial regeneration mechanism extending to other epithelia beyond the bile ducts.
In parallel, we use patient-derived cholangiocyte organoids to model biliary diseases in the lab, understand their pathophysiology and apply this knowledge to develop new therapeutics.
Finally, we are using primary cholangiocyte organoids to develop regenerative therapies for cholangiopathies. We use 2 complementary approaches: Direct organoid injection in diseased bile ducts as a cellular therapy; or the generation of bioengineered bile ducts which can be used to surgically replace damaged ducts.
Plain English
The bile ducts form a network of tubes in the liver draining bile, which is a toxic digestive fluid, into the intestine. In biliary diseases or cholangiopathies, the bile resistant lining of these tubes – made up of cells called cholangiocytes- breaks down, toxic bile overflows into the liver and causes liver failure and ultimately death. Normally, when cholangiocytes break down they quickly regenerate, and prevent significant leakage of bile into the surrounding liver. However, this mechanism fails in disease. We use lab-grown ducts-in-a-dish to understand why this happens, apply the resulting knowledge to restore cholangiocyte regeneration and reverse duct damage in biliary disorders. In parallel, we explore whether we can use our mini-ducts or even human-size ducts (bioengineered bile ducts) generated in the lab to repair or replace damaged ducts and reverse the damage caused by cholangiopathies
Key publications
1. Brevini, T., Maes, M., Webb, G.J. et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 615, 134–142 (2023). https://doi.org/10.1038/s41586-022-05594-0
2. Sampaziotis F, Muraro D, ……, Vallier L. Cholangiocyte organoids can repair bile ducts after transplantation in human liver. Science. 2021;371:839-846.
3. Tysoe OC, Justin AW, Brevini T, Chen SE, Frank A, Melum E, Saeb-Parsy K*, Markaki AE*, Vallier L*, Sampaziotis F*. Isolation and propagation of primary human cholangiocyte organoids for the generation of bio-engineered biliary tissue. Nat Protoc. 2019;14(6):1884-1925.
4. Sampaziotis F, et al. Extrahepatic cholangiocyte organoids for cell-based therapy applications. Nat Med. 2017;23:954-963.
5. Sampaziotis F, et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc. 2017;12:814-827.
6. Sampaziotis F, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845-52.