Dr Emma Rawlins
Stem cell fate in the mammalian lung
Email: e.rawlins@gurdon.cam.ac.uk
Departmental Affiliation: Gurdon Institute
Research
From first breath to last gasp, our lungs are an essential organ. Lung architecture is complex and must be maintained throughout life. If things go wrong with lung maintenance, the resulting changes can contribute to multiple different lung diseases. Many of these are degenerative diseases – such as Chronic Obstructive Pulmonary Disease – and are associated with ageing. Consequently, they are increasing in prevalence worldwide. In common with other organs, the lung is maintained by the function of tissue-specific stem cells which must act on demand to replace old or dying cells. Specifically, the stem cells must do two things:
- produce new daughter cells when required to do so: either too few or too many cells can be disastrous for lung function.
- produce the correct types of daughter cells: changes to cell identity can also disrupt lung function.
The Rawlins lab investigates the mechanisms which control stem cell behaviour in the developing and adult lungs. We are most interested in how the stem cells in the normally-developing human lung know which type of daughter cell they need to make and when. Our approach is to combine human organoid technology with the power of mouse genetics to understand the control of lung stem cell behaviour at the single cell level. This allows individual cells to be analyzed quantitatively in vivo, or by live-imaging in organoid and organ culture systems. One current interest is dissecting the molecular and cellular mechanisms which control a multipotent human lung epithelial stem cell during normal development.
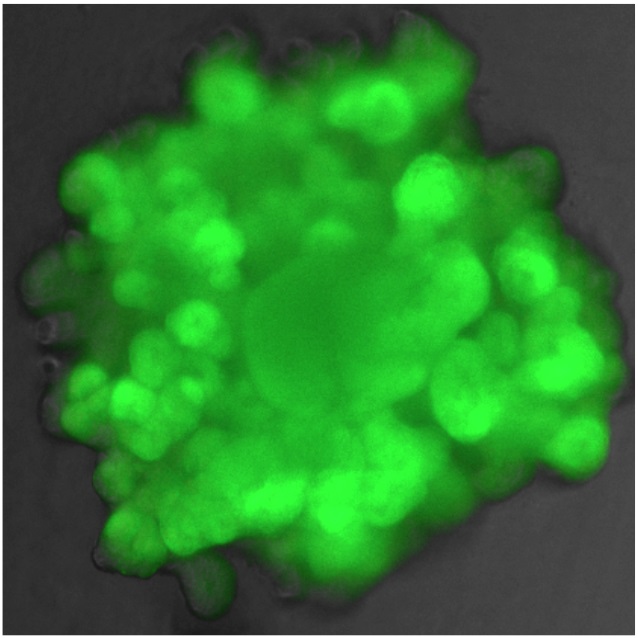
Fluorescent reporter lung organoid - Dr Marko Nikolić
Proof of principle that modifying organoid genomes is possible. A human embryonic lung organoid with Green Fluorescent Protein expressed in every cell.
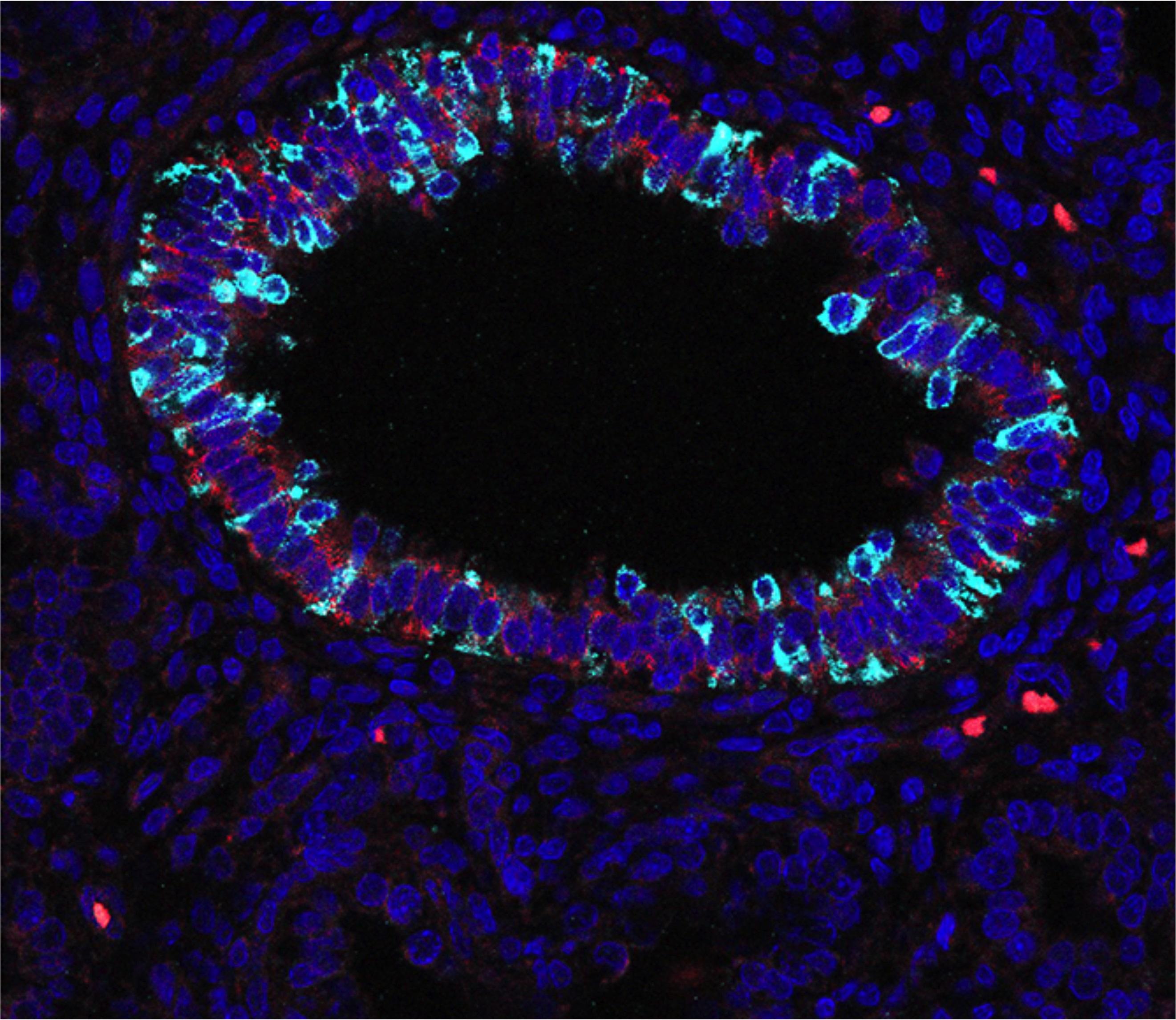
Differentiating human airway epithelium. - Dr Kyungtae Lim
Selected publications:
-
Lim K, Donovan A, Tang W, Sun D, He P, Teichmann SA, Marioni JC, Meyer KB, Brand AH, Rawlins EL (2023) Organoid modelling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell, 30: 20-37, doi.org/10.1016/j.stem.2022.11.013
-
He P, Lim K, Sun D, Pett JP, Jeng Q, Polanski K, Dong Z, Bolt L, Richardson L, Mamanova L, Dabrowska M, Wilbrey-Clark A, Madissoon E, Tuong ZK, Dann E, Suo C, Kai’En IG, He X, Barker B, Teichmann SA, Marioni J, Meyer KB, Rawlins EL. (2022) A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 185: 4841-4860, doi.org/10.1016/j.cell.2022.11.005
-
Sun D, Batlle OL, van den Ameele J, Thomas C, He P, Lim K, Tang W, Xu C, Meyer KB, Teichmann SA, Marioni J, Jackson SP, Brand AH, Rawlins EL (2022) SOX9 maintains human foetal lung tip progenitor state by enhancing WNT and RTK signalling. EMBOJ e111338, doi.org/10.15252/embj.2022111338
- Nikolić MZ, Sun D and Rawlins EL (2018) Human lung development: recent progress and new challenges. Development 145, dev163485. doi:10.1242/dev.163485 (Review article)
- Nikolić MZ, Caritg O, Jeng Q, Johnson J, Sun D, Howell KJ, Brady JL, Laresgoiti U, Allen G, Butler R, Zilbauer M, Giangreco A, Rawlins EL (2017) Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife Jun 30;6. pii: e26575. DOI: 10.7554/eLife.26575. PMC5555721.
- Balasooriya GI, Goschorska M, Piddini E, Rawlins EL (2017) FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development 144, 1600-1606. PMC5450841.
Additional links


