Professor Elisa Laurenti
Human haematopoietic stem cells biology in health and disease
Email: el422@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Haematology
Biography
Elisa Laurenti received her Masters in Biological Sciences from the University of Bologna in 2003. She then undertook her PhD under the supervision of Prof. Andreas Trumpp at the Swiss Institute for Cancer Research in Lausanne, Switzerland. In 2010 she joined Dr John Dick’s laboratory at University Health Network (Toronto, Canada) where she became interested in the study of human hematopoietic stem cells.
In 2014, she established her own research group at the Cambridge Stem Cell Institute and Department of Haematology of the University of Cambridge. She is currently a Wellcome Trust and Royal Society Sir Henry Dale Research Fellow. In 2021, she received the International Society for Experimental Hematology Janet Rowley Award which recognises outstanding early career principal investigators and their contributions to the field of haematology.
Elisa is the Academic Champion for Public Engagement at the Cambridge Stem Cell Institute and chairs the Public Engagement Steering Committee.
Funding
Wellcome, Royal Society, BBSRC, Medical Research Council, Kay Kendall Leukaemia Fund, Birax
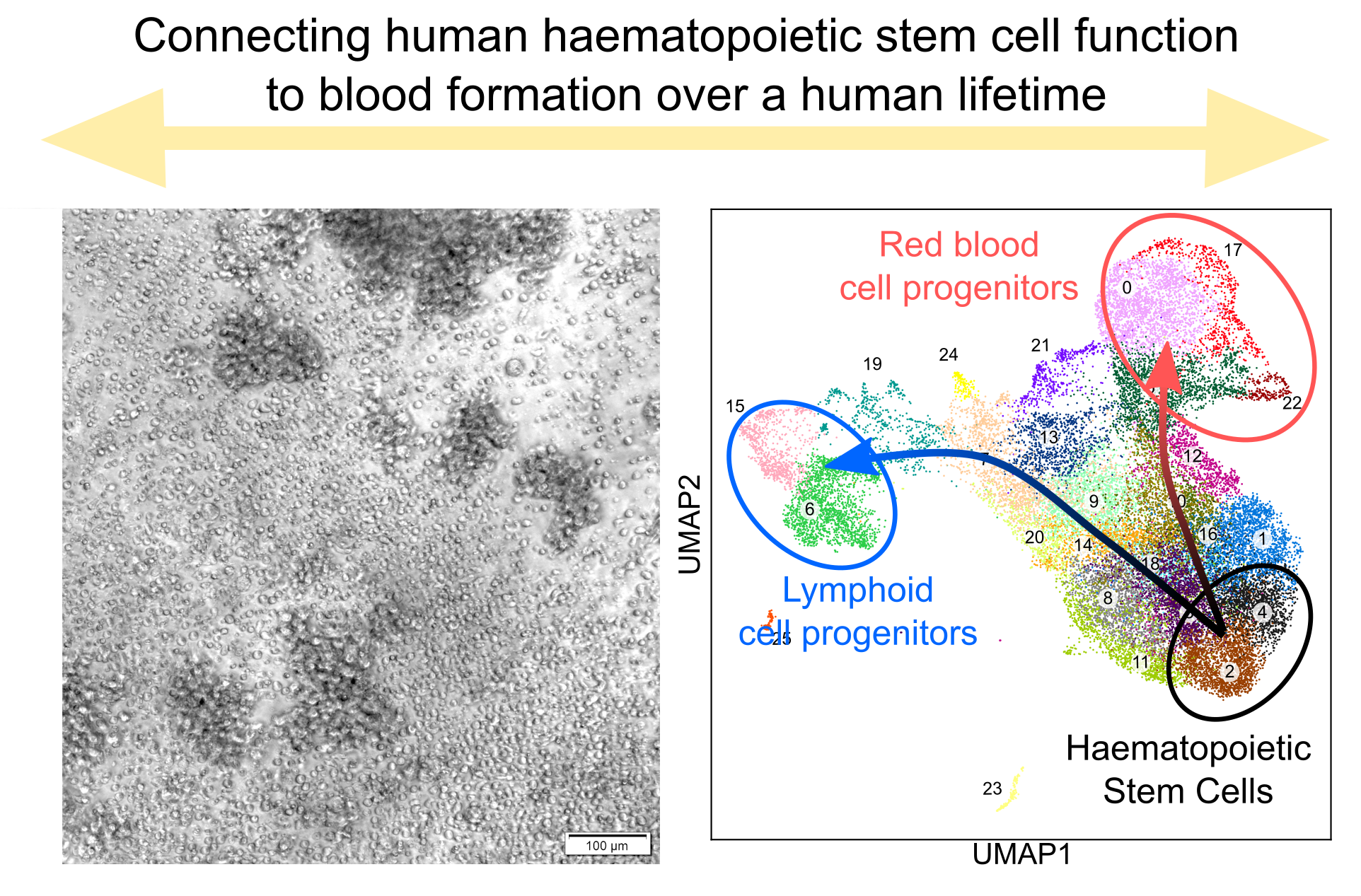
Left: image of different blood cell types originated from a single human haematopoietic stem cell in vitro (credit: Aditi Vedi); Right: the transcriptional landscape of the human haematopoietic hierarchy, derived from single cell transcriptomics (credit: Hugo Bastos, Nicole Mende).
Research
The human body makes one trillion blood cells per day. These include red blood cells, platelets and immune cells, which all provide vital physiological functions. Rare blood stem cells, also called haematopoietic stem cells (HSCs) are responsible for life-long blood production. Their function must be tightly regulated to continuously produce blood in accordance with the demands of our organism throughout our lifespan, while also ensuring recovery from infections and injuries. HSC are very different from all other blood cell types and have unique functional properties, such as their infrequent division and their capacity to give rise to all blood cell types.
The Laurenti laboratory aims to understand blood formation throughout a human lifetime. The lab studies human HSC and progenitor cells using single cell biology techniques: functional single cell assays and single cell -omics technologies. The lab is currently investigating the unique molecular and functional properties of human HSC and progenitors during foetal life, throughout adulthood across sites of haematopoiesis as well as during ageing. Another area of interest is the study of HSCs’ cellular and molecular responses to stress, with the goal to improve HSC ex vivo expansion and gene therapy protocols.
Understanding how the cellular and molecular composition of the HSC/progenitor compartment changes in stress conditions and throughout a human lifetime has important implications for regenerative medicine and treatment of blood cancers.
Plain English
Every day a trillion blood cells are produced in our body. This amazing turnover is achieved thanks to blood stem cells. These cells have the unique capacity to produce all blood cells in healthy individuals but also after injury or infection. Their function is vital, because if impaired, either blood production fails or cancer arises. Our laboratory thus focuses on these 2 important questions:
- How are human blood stem cells different from other blood cell types?
- What is the impact of age and disease on human blood stem cell function? For this we measure human blood stem cell responses to a number of signals, compare them to other blood cells and identify specific genes that regulate their behaviour. We also study what goes wrong in blood stem cells when there is inflammation, in the elderly and during the early stages of cancer development. Understanding how blood formation occurs in humans in all of these contexts we hope will help design new therapies against a range of diseases.
Datasets
Find more description of the lab's published scRNAseq datasets (Mende et al., Blood, 2022) and user-friendly web apps to browse the data below.
- The ShinyApp for exploring the scRNA-seq landscapes comparing healthy BM, spleen and non-mobilised PB is accessible via this link.
- The link to access the scRNA-seq landscapes comparing G-CSF mobilised PB to BM and non-mobilised PB is accessible here.
Laurenti Group photo
Key Publications
-
Mitchell E, Chapman MS, Williams N, Dawson K, Mende N, Calderbank EF, […] Saeb-Parsy K, Green A, Nangalia J*, Laurenti E*, Campbell P*, Clonal dynamics of haematopoiesis across the human lifespan, Nature, in press
-
Mende N, Bastos HP, Santoro A, Mahbubani KT, Ciaurro V, Calderbank EF, Quiroga Londoño M, Sham K, Mantica G, Morishima T, Mitchell E, Lidonnici MR, Meier-Abt F, Hayler D, Jardine L, Curd A, Haniffa M, Ferrari G, Takizawa H, Wilson NK, Gottgens B, Saeb-Parsy K, Frontini M, Laurenti E. Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans. Blood. 2022 Jan 24:blood.2021013450. https://doi.org/10.1182/blood.2021013450
- Popescu D., Botting R.A., Stephenson E., Green K., Webb, S., Jardine L., Calderbank E.F., […] Regev A.D., Chedotal A., Roberts I., Gottgens B., Behjati S.*, Laurenti E.*, Teichmann S.D.*, Haniffa M.*, Decoding human fetal liver haematopoiesis. Nature, 574, 2019, 365-371.
- Belluschi, S.*, Calderbank, E.F.*, Ciaurro, V., Pijuan-Sala, B., Santoro, A., Mende, N., Diamanti, E., Sham, K.Y.C., Wang, X., Lau, W.W.Y., Jawaid W., Göttgens B., Laurenti E., Myelo-lymphoid lineage restriction occurs in the human haematopoietic stem cell compartment before lymphoid-primed multipotent progenitors. Nature Communications, 2018, 9, 4100.
-
Laurenti E & Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418 (2018) PMID:29364285
-
Laurenti E*, Frelin C*, Xie S*, Ferrari R, Dunant CF, Zandi S, Neumann A, Plumb I, Doulatov S, Chen J, April C, Fan J-B, Iscove N, Dick JE. CDK6 Levels Regulate Quiescence Exit in Human Hematopoietic Stem Cells. Cell Stem Cell, 2015 Feb 19; 16 (3):302-313. PMCID:PMC4359055
* Equal contribution by these authors



