Professor Ragnhildur Thóra Káradóttir
Neurotransmitter signalling to central nervous system progenitor cells
Email: rk385@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Veterinary Medicine
Biography
Ragnhildur Thóra Káradóttir, currently the director of the MS Society Cambridge Centre for Myelin Repair, did her undergraduate degree in Biochemistry at the University of Iceland. For her postgraduate training, she entered the Wellcome Trust 4 year PhD Programme in Neuroscience, at UCL, where she did her PhD with Prof. David Attwell. Immediately, after her PhD she was awarded a Dorothy Hodgkin Fellowship of the Royal Society, and in 2011 she was awarded a Wellcome Trust Career Development Research Fellowship.
Since establishing her lab she has been awarded a number of awards, most recently the Lister Institute Research Prize (one of 5 in the UK), the Allen Distinguished Investigator Award (one of 5 worldwide, first time given outside of USA) and an ERC consolidator award. In 2015 she was elected to the FENS-Kavli Network of Excellence (one of 20 in Europe) and in 2017 awarded the Fabiane Carvalho Miranda International Prize for the best paper published in the years 2015-2017 in myelin biology and MS related research.
Her main research interest is to understand how neuronal activity can regulate oligodendrocyte precursor cells (OPCs) differentiation and myelin plasticity in health and disease. Her new line of research interest is to determine the changes in myelin and myelin repair throughout the lifespan.
Education
- 2006 Doctor of Philosophy (PhD) in Neuroscience from University of London.
- Thesis title: Neurotransmitter signalling to oligodendrocytes
- 2001 Admission to the 4-year PhD Programme in Neuroscience at University College London
- 2000 BSc in Biochemistry from the University of Iceland, 1st Class. Research project: Role of melatonin in Seasonal Affective Disorder
Professional History
- 2021 Director of the Cambridge Centre for Myelin Repair
- 2011 Wellcome Trust Career Development Research Fellow
- 2007 Dorothy Hodgkin Research Fellow of the Royal Society held in Dept. of Veterinary Medicine University of Cambridge
- 2006 Postdoctoral research fellow with Prof. David Attwell, Dept. Physiology University College London
- 2001- Wellcome Trust PhD student on 4-year PhD in Neuroscience, Department of Physiology 2005 University College London
Funding
MS Society, Paul G Allen Family Foundation, Lister Institute, Wellcome, BBSRC, ERC
External links
www.vet.cam.ac.uk/directory/rk385@cam.ac.uk
https://www.neuroscience.cam.ac.uk/directory/profile.php?rk385
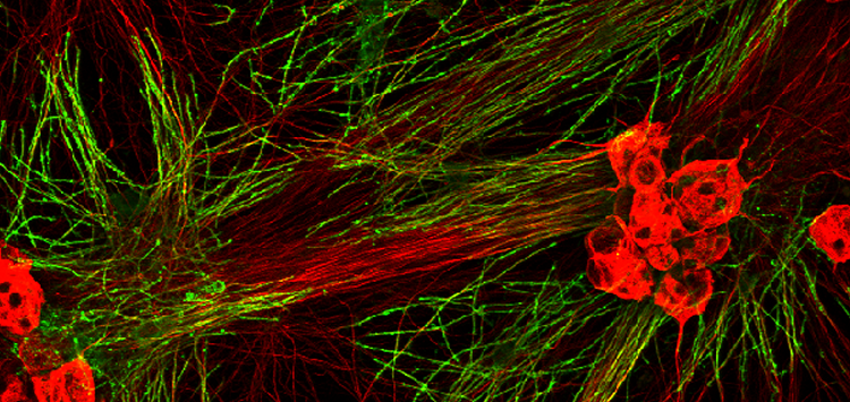
White matter in a dish! Myelinating co-culture where oligodendrocyte precursor cells (that are plated on top of DRG axons) differentiate into myelinating oligodendrocytes (green; MBP) that myelinate the DRG axons (red; neurofilament). Credit Kimberley Evans
Research
The CNS white matter links billions of neurons in the grey matter. Its function depends on oligodendrocytes enwrapping neuronal axons with myelin to synchronize and increase information flow between neurons: essential for our cognitive abilities, our perception of the world and our motor skills. The importance of myelin becomes evident in diseases, such as multiple sclerosis, where myelin damage leads to cognitive and motor disability. Unique to the CNS, myelin regeneration can occur spontaneously in demyelinating disease, as adult oligodendrocyte precursor cells (OPCs; a CNS stem cell that comprises 5% of all cells in the brain) respond to the demyelinating injury and differentiate into new myelinating oligodendrocytes. However, this process often fails, making OPCs differentiation an important therapeutic target.
The Karadottir lab has previously shown that OPCs express neurotransmitter receptors and receive synaptic inputs from neuronal axons in the white matter, hence are capable of sensing changes in neuronal activity. The lab’s interest is to understand how signals from neurons induce OPCs to differentiate and myelinate axons during development and with normal ageing; this also could be an underlying mechanism for white matter plasticity.
The devastating consequences of dys/demyelination, in diseases like cerebral palsy, spinal cord injury and multiple sclerosis makes it important to study how OPCs differentiation is regulated. They are actively investigating how OPCs respond to myelin injury and whether neuronal activity and neurotransmitter signalling may regulate the myelin repair process. The lab’s ultimate aim is to find new treatments for white matter disease.
 Karadottir Group photo
Karadottir Group photo
Plain English
For our brain to work, fast electrical communication between nerve cells is essential. This is achieved by insulating the nerves with a fatty substance called myelin. In diseases like multiple sclerosis, spinal cord injury and stroke, myelin is lost, while in cerebral palsy myelin fails to develop. Lack of myelin causes physical and mental disability. Myelin is provided by cells called oligodendrocytes, which develop from oligodendrocyte precursor cells (OPCs). In the adult, OPCs can repair myelin, but this repair often fails for reasons currently unknown. OPCs can also develop into other types of brain cell, including nerve cells, but it is not known what controls this choice of cell identity. We study how OPCs generate myelin during development and in disease. By investigating how signals in the cells’ environment interact with the properties of the OPCs to instruct them to migrate, generate myelin-making oligodendrocytes, or develop into other brain cells. The aim of this work is to understand how OPCs decide to become myelinating cells, how we can influence them to repair myelin in disease.
Key Publications
- Monje M, Káradóttir RT. (2020) The bright and the dark side of myelin plasticity: Neuron-glial interactions in health and disease. Semin Cell Dev Biol :S1084-9521(20)30183-X.
- Bonetto G, Kamen Y, Evans KA, Káradóttir RT. (2020) Unraveling Myelin Plasticity. Front Cell Neurosci. 11;14:156. PMCID: PMC7301701
- Neumann B, Foerster S, Zhao C, Bodini B, Reich DS, Bergles DE, Káradóttir RT, Lubetzki C, Lairson LL, Zalc B, Stankoff B, Franklin RJM. (2020) Problems and Pitfalls of Identifying Remyelination in Multiple Sclerosis. Cell Stem Cell 26(5):617-619. doi: 10.1016/j.stem.2020.03.017.
- Jia W, Kamen Y, Pivonkova H, & Káradóttir R. (2019) Neuronal activity-dependent myelin repair after stroke Neuroscience. 703:139-144.
- Spitzer S, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O, Agathou S & Káradóttir R. (2019) Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron,101(3):459-47. PMCID: PMC6372724
- Agathou S & Káradóttir R (2019) Whole-cell patch clamp recordings from oligodendrocyte lineage cells in brain slices. In D. Lyons D, and L. Kegel (Eds.), Methods in Molecular Biology: Oligodendrocytes: Methods and Protocols (pp. 1141-168). Springer Nature. doi: 10.1007/978-1-4939-9072-6_9.
- Chorghay Z, Káradóttir R, Ruthazer ES (2018). White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap. Front Cell Neurosci, 12:428. PMCID: PMC6251003
- Kuo CT & Káradóttir R (2018). Neuronal Activity-Dependent Control of Postnatal Neurogenesis and Gliogenesis. Annual Reviews in Neuroscience, 41:139-161. PMCID: PMC6324739
- Chen Y, Spitzer S, Agathou S, Karadottir RT, Smith A. (2017) Gene Editing in Rat Embryonic Stem Cells to Produce In Vitro Models and In Vivo Reporters. Stem Cell Reports 9(4):1262-1274. PMCID: PMC5639479
- de Faria Jr O, Pama EAC, Evans KA, Luzhynskaya A & Káradóttir R (2017) Neuroglial interactions underpinning myelin plasticity. Dev. Neurobiology, 78(2):93-107.
- Stockley JH, Evans KA, Matthey M, Volbracht K, Agathou S, Mukanowa J, Burrone J & Káradóttir R. (2017) Surpassing light-induced cell damage in vitro with novel cell culture media. Science Reports 7: 849. PMCID: PMC5429800
- Gautier HO, Evans K, Lundgaard I, James F, Lao-Peregrin C, Franklin RJM, Káradóttir R (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nature Communications 6: 8518. PMCID:PMC4600759
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJM, ffrench-Constant C, Attwell D, Káradóttir R (2013). Neuregulin and BDNF induce a switch to NMDA receptor dependent myelination by oligodendrocytes. PLoS Biol. 11(12): e1001743. PMCID:PMC3876980
- Káradóttir R, Hamilton N, Bakiri Y & Attwell D (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nature Neuroscience 11(4): 450-456. PMCID:PMC2615224
- Káradóttir R, Attwell D (2006). Combining patch-clamping of cells in brain slices with immunocytochemical labelling to define cell type and developmental stage. Nature Protocols 1(4): 1977-1985. PMCID:PMC2682777
- Káradóttir R, Cavalier P, Bergersen LH, Attwell D (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438: 1162-1166. PMCID:PMC1416283


