Dr Daniel Hodson
Deciphering the genomics of B cell lymphomas
Email: djh1002@cam.ac.uk
Laboratory: Cambridge Stem Cell Institute, Jeffrey Cheah Biomedical Centre
Departmental Affiliation: Haematology
Biography
Daniel Hodson studied Medicine at Cambridge University and then clinical medicine at Oxford University. He subsequently trained as a clinical haematologist with a special interest in lymphoid malignancies. During his haematology training he undertook a PhD in molecular immunology at the Babraham Institute in Cambridge under the supervision of Dr Martin Turner, where he studied the contribution of post-transcriptional regulation to the normal lymphocyte development. In 2010 he moved to the National Cancer Institute, USA, as a post-doctoral fellow in the lab of Dr Lou Staudt, where he developed expertise in the application of functional genomics to the study of B cell lymphomas. In 2015 he returned to Cambridge as a Medical Research Council Clinician Scientist and group leader in the SCI and the Department of Haematology. In 2021 he was awarded a CRUK Senior Cancer Research Fellowship. His group researches the molecular mechanisms that underlie lymphomagenesis. Dr Hodson also holds an honorary consultant contract in the Haematology Department at Cambridge University NHS Hospitals Trust.
Funding
CRUK, Kay Kendal Leukaemia Fund, Addenbrooke's Charitable Trust, The Evelyn Trust, MRC
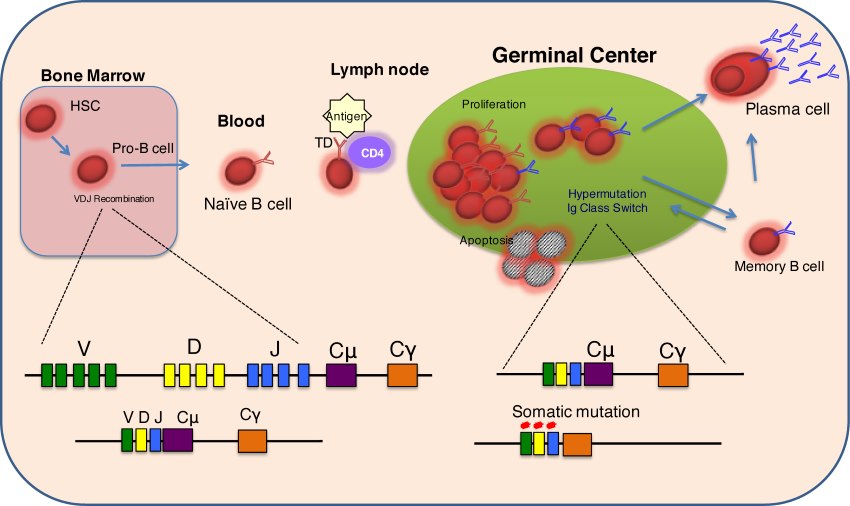
The unique and dangerous life of the B cell
Research
Normal B lymphocytes progress through a series of developmental stages that begin with the haematopoietic stem cell. Progression through each of these stages is tightly controlled at both the transcriptional and post- transcriptional levels. Genetic alterations and mutations, which can occur at any stage from the haematopoietic stem cell to the post-germinal centre B cell, can lead to loss of this normal regulation and subsequently to the development of lymphoid malignancies such as non-Hodgkin Lymphoma (NHL), which is the 6th commonest form of human cancer.
Understanding how these genetic alterations corrupt cell fate choices at each stage of lymphocyte development will be the key to identifying cellular pathways that can be therapeutically targeted. The Hodson Group is developing novel cell culture models to study the effects of these genetic alterations in human lymphocytes. In particular, they are interested in studying how these genetic alterations lead to changes at the level of mRNA translation and how these posttranscriptional changes then contribute to lymphomagenesis.
The lab uses a variety of techniques including exome and RNA sequencing, ribosome profiling, iCLIP and single cell perturbational transcriptomics to identify the developmental timing of these genetic alterations, their mechanistic contribution to lymphomagenesis and the implications this has for the treatment and monitoring of patients.
Hodson Group photo
Key Publications
- Gong C, Krupka JA, Gao J, Grigoropoulos NF, Giotopoulos G, Asby R, Screen M, Usheva Z, Cucco F, Barrans S, Painter D, Zaini NBM, Haupl B, Bornelöv S, Ruiz De Los Mozos I, Meng W, Zhou P, Blain AE, Forde S, Matthews J, Khim Tan MG, Burke GAA, Sze SK, Beer P, Burton C, Campbell P, Rand V, Turner SD, Ule J, Roman E, Tooze R, Oellerich T, Huntly BJ, Turner M, Du MQ, Samarajiwa SA, Hodson DJ. Sequential inverse dysregulation of the RNA helicases DDX3X and DDX3Y facilitates MYC-driven lymphomagenesis. Mol Cell. 2021 Oct 7;81(19):4059-4075. PMID: 34437837
-
Morin RD, Arthur SE, Hodson DJ. Molecular profiling in diffuse large B-cell lymphoma: why so many types of subtypes? Br J Haematol. 2021 Aug 31. PMID: 34467527
-
Gao J, Sidiropoulou E, Walker I, Krupka JA, Mizielinski K, Usheva Z, Samarajiwa SA, Hodson DJ. SGK1 mutations in DLBCL generate hyperstable protein neoisoforms that promote AKT independence. Blood. 2021 Sep 16;138(11):959-964. PMID: 33988691
- Caeser R, Gao, J, Di Re M, Gong J & Hodson DJ. Genetic manipulation and immortalized culture of ex vivo primary human germinal center B cells. Nature Protocols. 2021 Apr 9. doi: 10.1038/s41596-021-00506-4. Online ahead of print. PMID: 33837304
- Lacey S, Barrans S, Beer P, Painter D, Smith A, Roman E, Cooke S, Ruiz C, Glover P, Van Hoppe S, Webster N, Campbell P, Tooze R, Patmore R, Burton C, Crouch S & Hodson DJ. Targeted sequencing in DLBCL, molecular subtypes and outcomes: a Haematological Malignancy Research Network report. Blood. 2020 May 14;135(20):1759-1771. PMID: 32187361
- Caeser R, Di Re M, Krupka JA, Gao J, Lara-Chica M, Dias J, Cooke S, Fenner R, Usheva Z, Runge H, Beer PA, Eldaly H, Pak HK, Park CS, Vassiliou G, Huntly BJP, Mupo A, Bashford-Rogers RJM & Hodson DJ. Genetic modification of primary human B cells generates translationally-relevant models of high-grade lymphoma. Nature Communications. 2019 Oct 4;10(1):4543. PMID: 31586074



