Submitted by Administrator on Mon, 03/07/2017 - 12:22
Scientists at the Cambridge Stem Cell Institute and the Sanger Institute have developed a new method for growing and transplanting artificial bile ducts that could in future be used to help treat liver disease in children, reducing the need for liver transplantation.
In research published in the journal Nature Medicine, the researchers grew 3D cellular structure which, once transplanted into mice, developed into normal, functioning bile ducts.
Bile ducts are long, tube-like structures that carry bile secreted by the liver and essential for helping us digest food. If the ducts do not work correctly, for example in the childhood disease biliary atresia, this can lead to damaging build of bile in the liver and the need for a liver transplant.
The study suggests that it will be feasible to generate and transplant artificial human bile ducts using a combination of cell transplantation and tissue engineering technology. This approach provides hope for the future treatment of diseases of the bile duct; at present, the only option is a liver transplant.
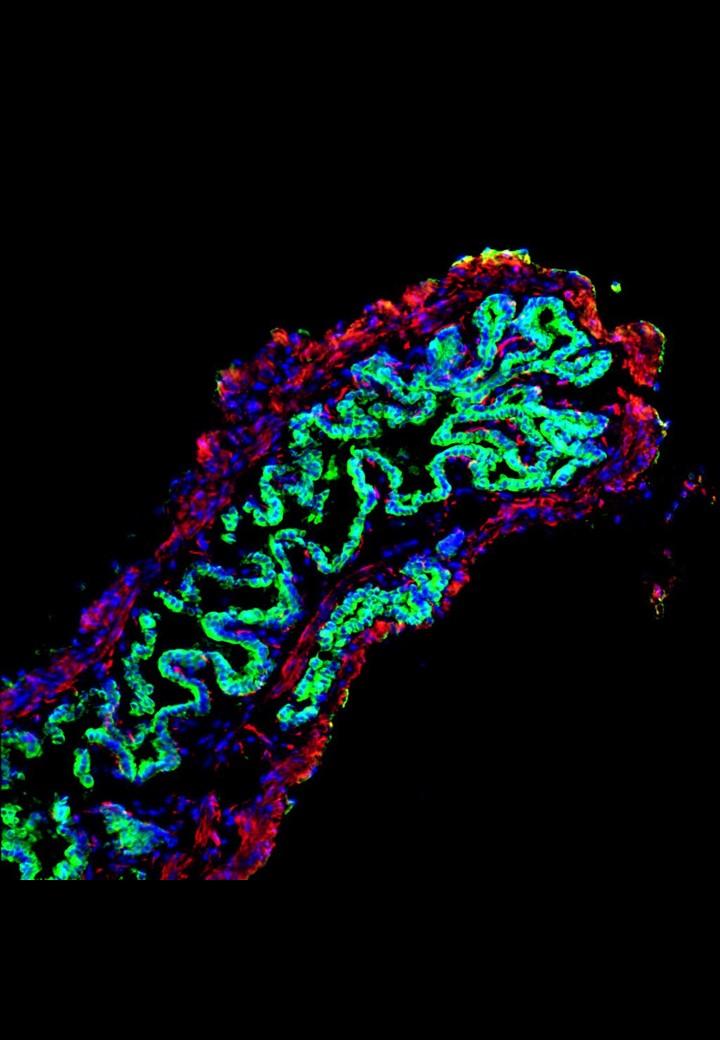
The research team, led by Professor Ludovic Vallier and Dr Fotis Sampaziotis from the Wellcome – MRC Cambridge Stem Cell Institute and the Wellcome Trust Sanger Institute, and Dr Kourosh Saeb-Parsy from the Department of Surgery, extracted healthy cells (cholangiocytes) from bile ducts and grew these into functioning 3D duct structures known as biliary organoids. When transplanted into mice, the biliary organoids assembled into intricate tubular structures, resembling bile ducts.
The researchers, in collaboration with Mr Alex Justin and Dr Athina Markaki from the Department of Engineering, then investigated whether the biliary organoids could be grown on a “biodegradable collagen scaffold”, which could be shaped into a tube and used to repair damaged bile ducts in the body. After 4 weeks, the cells had fully covered the miniature scaffolding resulting in artificial tubes which exhibited key features of a normal, functioning bile duct. These artificial ducts were then used to replace damaged bile ducts in mice. The artificial duct transplants were successful, with the animals surviving without further complications.
“Our work has the potential to transform the treatment of bile duct disorders,” explains Professor Vallier. “At the moment, our only option is liver transplantation, so we are limited by the availability of healthy organs for transplantation. In future, we believe it will be possible to generate large quantities of bioengineered tissue that could replace diseased bile ducts and provide a powerful new therapeutic option without this reliance on organ transplants.”
“This demonstrates the power of tissue engineering and regenerative medicine,” adds Dr Sampaziotis. “These artificial bile ducts will not only be useful for transplanting, but could also be used to model other diseases of the bile duct and potentially develop new drug treatments.”
Professor Vallier is part of the Department of Surgery at the University of Cambridge and his team are jointly based at the Wellcome Trust – MRC Cambridge Stem Cell Institute and the Wellcome Trust Sanger Institute.
The support of the Medical Research Council, Sparks children’s medical research charity and the European Research Council has been invaluable in facilitating this research, and is gratefully acknowledged by Ludovic and the team.
Read also:
Cambridge News http://www.cam.ac.uk/research/news/artificial-bile-ducts-grown-in-lab-and-transplanted-into-mice-could-help-treat-liver-disease-in.
The Naked Scientists article www.thenakedscientists.com/articles/science-news/new-bile-ducts-grown-dish
The Naked Scientists programme www.thenakedscientists.com/articles/interviews/growing-replacement-bile-ducts
Publication details:
Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, Gieseck RL, Cardoso de Brito M, Berntsen NL, Gómez-Vázquez MJ, Ortmann D, Yiangou L, Ross A, Bargehr J, Bertero A, Zonneveld MCF, Pedersen MT, Pawlowski M, Valestrand L, Madrigal P, Georgakopoulos N, Pirmadjid N, Skeldon GM, Casey J, Shu W, Materek PM, Snijders KE, Brown SE, Rimland CA, Simonic I, Davies SE, Jensen KB, Zilbauer M, Gelson WTH, Alexander G, Sinha S, Hannan NRF, Wynn TA, Karlsen TH, Melum E, Markaki AE, Saeb-Parsy K, Vallier L. Reconstruction of the murine extrahepatic biliary tree using primary extrahepatic cholangiocyte organoids. Nature Medicine. DOI: 10.1038/nm.4360

