Submitted by Laura Puhl on Mon, 07/03/2022 - 10:31
Research finds identifiers that can speed the process of in-vitro platelet production
A new paper from the Wellcome-MRC Cambridge Stem Cell Institute (CSCI) and the UK Regenerative Medicine Platform has identified previously unknown markers of cells called megakaryocyte progenitors. This discovery will lead to better understanding of these important intermediates in platelet production for transfusion, thereby improving the process in the future.
The research, published last month in Science Advances, investigated the differences between cell products grown in a lab and those grown in the human body in order to better understand how the lab process corresponds to how our body produces platelets.
Professor Cédric Ghevaert comments on the research, “The crux of the paper is using single sequencing technology to understand how stem cells become megakaryocytes, the cells we ultimately use to make platelets for transfusion. Crucially, we apply that knowledge to produce more of these cells, helping the process to become more efficient.”
Optimising the process
Platelets are produced by megakaryocytes (large bone marrow cells) and act as initiators for clotting at sites of vessel injury. For patients with a low platelet count - a condition known as thrombocytopenia, caused by cancer treatment, immune system issues or bleeding due to trauma or surgery - this shortage can lead to dangerous or life-threatening bleeding. Treatment for thrombocytopenia involves platelet transfusions, and every year in the UK 280,000 platelet units are utilised in this way.
However, while transfusions have traditionally relied on donors with matching blood types, people who have previously undergone transfusions or women who have had multiple children are at risk of becoming resistant to transfused platelet units. Further adding to the complexity of these transfusions, platelets have a relatively short shelf life, lasting only 5-7 days, which often leads to shortages (particularly during times when donors are difficult to come by, as in the ongoing pandemic).
The Ghevaert Lab (CSCI) has been working on lab-derived platelets for transfusions for many years in order to decrease dependency on donors and provide platelets for those who do not have a donor match. This paper, driven by Dr Moyra Lawrence and in collaboration with the Bioinformatics team and several of CSCI’s PhD students, furthers the lab’s research by identifying previously unknown markers for cells that are essential for platelet production.
Using single-cell RNA sequencing and machine learning approaches, the team mapped the differentiation process of megakaryocytes, both to better understand the process and to ultimately optimise the production in vitro.
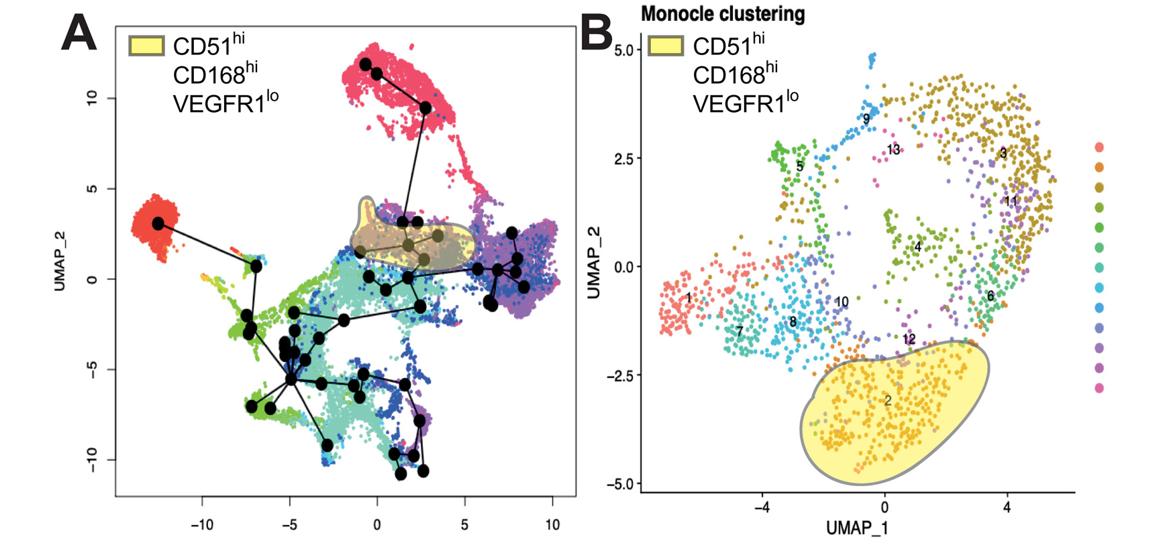
Figure 5 from the research paper shows the mapping process of the megakaryocyte progenitors.
Dr Lawrence comments, “Cell surface markers are structures on the outside of the cell which allow us to monitor cell types as they emerge. In this paper, we find five surface markers which can allow us to purify megakaryocyte progenitors and compare them to megakaryocyte progenitors in the human body. We were most surprised to find that our protocol made megakaryocytes in an accelerated manner, bypassing some intermediate cell types.”
As a result of this research, the scientists will be able to increase the efficiency of the platelet-production process, as well as enhance the longevity of cultures generating platelets for transfusions, potentially enabling the large-scale manufacturing of platelets for clinical purposes.
In the future, there is hope that currently difficult-to-match patients with thrombocytopenia will have greater access to platelets for their transfusions, and that scientists can apply this understanding to future research. Dr Lawrence says, “Together this work could allow us to produce platelets for transfusion more efficiently and understand how megakaryocyte progenitors maintain their stemness in the bone marrow.”
Learn more about laboratory-grown cells in Professor Ghevaert's video below: