Submitted by Greg Palmer on Wed, 24/01/2018 - 17:44
Scientists from the University of Cambridge and the Université Libre de Bruxelles have used cutting-edge technology to show for the first time how embryonic stem cells diversify to generate the progenitor cells required for the development of the heart.
The researchers hope that this new understanding will pave the way for future therapeutic opportunities for infants born with heart defects and other heart diseases.
Stem cells in the developing embryo have the extraordinary ability to become any type of cell in the body, with the process of development following set pathways controlled by our genes. The heart is the first organ to develop in the embryo and strict genetic instructions control the production of the different cell types that allow the heart to function (such as cardiomyocytes that control the pumping of the heart and pacemaker cells that control the timing of the heartbeat). If this process is disrupted severe heart defects occur including congenital heart disease which affects 9 in every 1000 infants born in the UK.
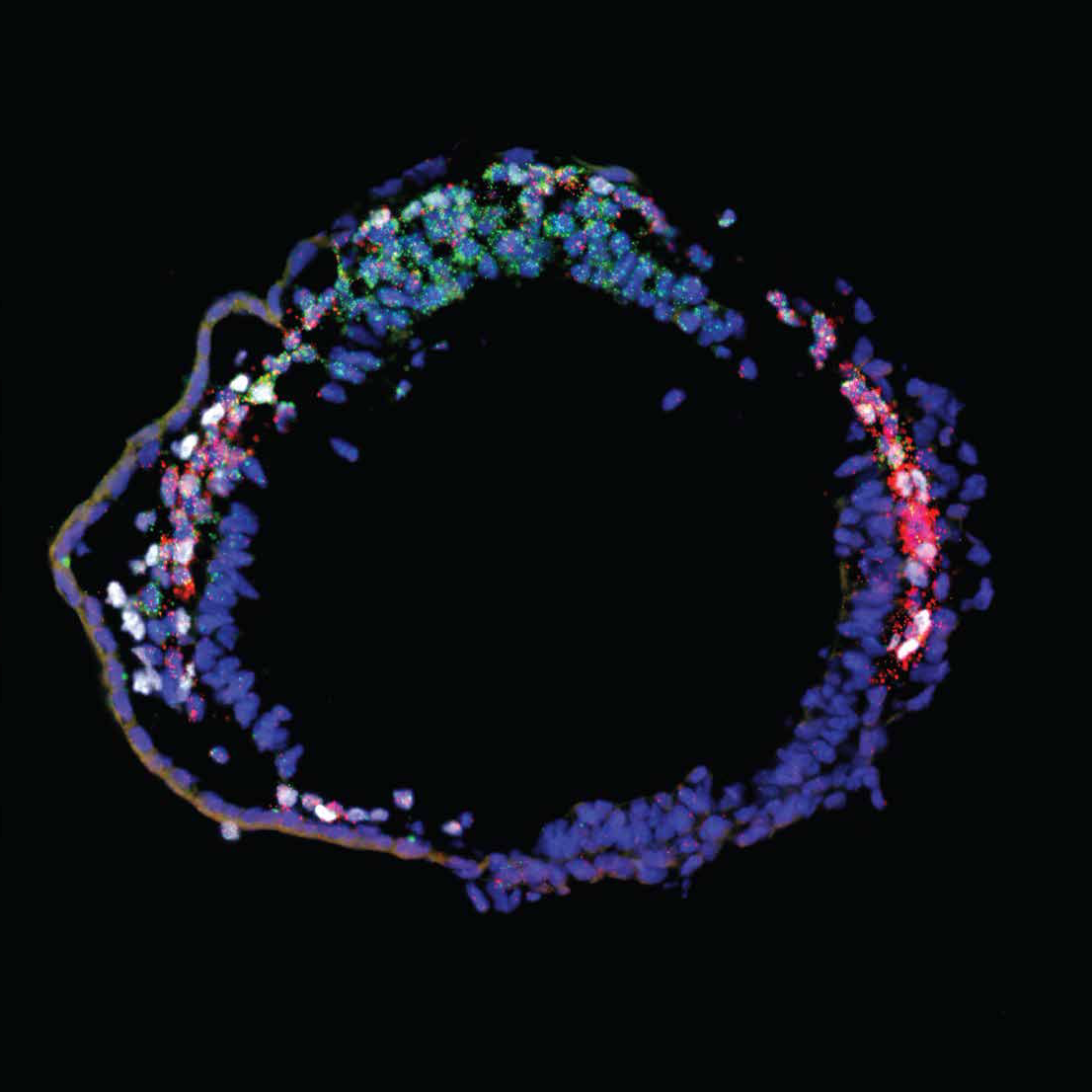
New research, published in the journal Science, used pioneering single-cell technology to study the genetic activity of the earliest heart progenitor cells present in the mouse embryo. The scientists took advantage of a cell labelling strategy based on a gene called Mesp1, which is critical for directing stem cells to become cardiac cells in the developing heart. Using powerful computer analysis, the researchers could pinpoint the distinct progenitor cell populations that would go on to develop into the specific cell types required for a fully functioning heart. The results also showed that development of the different cell populations begins at different time points, and that they migrate through specific locations in the early embryo.
“This new analysis shows that cardiovascular stem cells in the embryo are already “primed” to give rise to all the different types of heart cells, such as cardiac muscle cells or pacemaker cells” explains Professor Cédric Blanpain, one of lead researchers involved in the study. “Understanding the genetic features associated with early cardiovascular cell lineage commitment will be important in designing new strategies to manage and treat congenital heart disease”.
Professor Göttgens continued, stating “Our new discoveries critically depend on recent technological innovations that now allow us to determine the gene activity in individual single cells. Not only can we study tiny cell populations which wasn’t possible before, but we can also use the computer to separate the individual single cells into subgroups or cell types, based on their gene activity profiles. From these newly discovered gene profiles, we can discover new candidate genes that may be exploited for developing new therapies to repair the heart”.
Dr Nathan Richardson, MRC Head of Molecular and Cellular Medicine, said: “These new findings provide exciting insights into the complex development of the heart from progenitor cells. Crucially they also open the way for opportunities to develop new treatments for heart disease, and demonstrate the value of the MRC’s long-term commitment to stem cell research and discovery science.”
The researchers now plan to continue this cutting-edge research to discover the precise molecular defects that occur during the earliest stages of heart development in models of congenital heart disease. This they hope will pinpoint new targets for developing better therapies to repair and regenerate the heart.
Defining the earliest step of cardiovascular lineage segregation by single cell RNA-seq. F. Lescroart, X. Wang, X. Lin, B. Swedlund, S. Gargouri, A. Sànchez-Dànes, V. Moignard, C. Dubois, C. Paulissen, S. Kinston, B. Göttgens and C. Blanpain is published in Science.
The support of the Wellcome, MRC, Bloodwise, Cancer Research UK, National Institutes of Health, FNRS, the ULB foundation, the foundation Bettencourt Schueller and the Leducq Fondation are gratefully acknowledged by the team.

