Submitted by Administrator on Mon, 08/01/2018 - 14:33
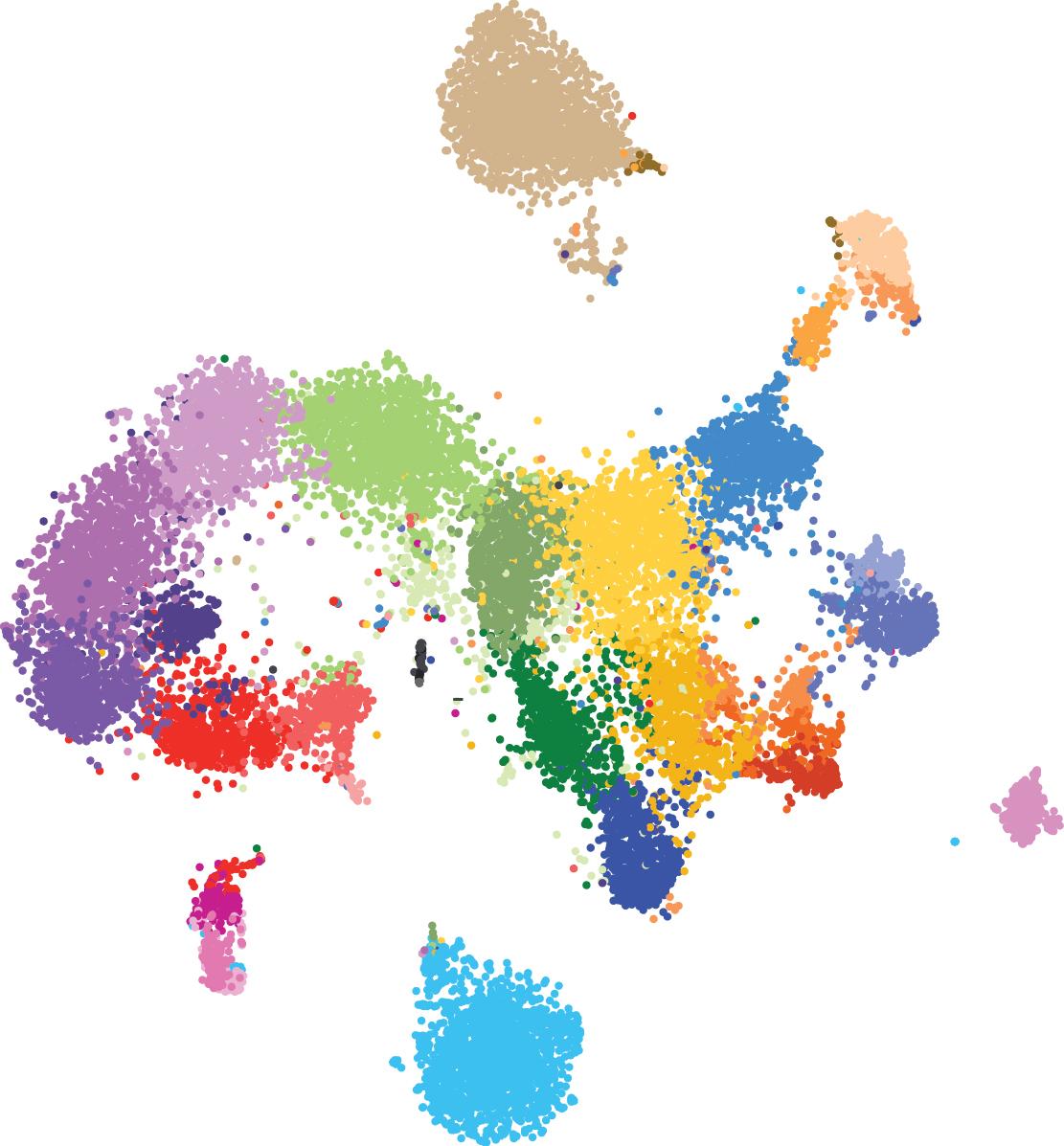
Cambridge scientists have used cutting-edge technology to profile over 20,000 individual cells from the developing embryo to produce the first ‘cell map’ describing all of the major cell types present in early embryo development. The researchers used the map to identify an important new pathway involved in blood cell development and say the map could open up new avenues for medicine and drug development.
The new research, published in the journal Nature Cell Biology, used pioneering single-cell technology to study the genetic activity of over 20,000 individual cells present in the mouse embryo at an early stage of development when major organs such as the heart and brain are forming. The patterns of genetic activity in the developing embryo were captured in the new ‘cell map’ that will help scientists understand how cells grow and acquire all the various specialized functions required for the body to function.
The Cambridge team demonstrated the utility of their platform by identifying a new pathway involved in early blood cell development. Blood cells mature in the embryo along with other tissues such as the heart, muscles, veins and arteries, however there are still major knowledge gaps in these areas.
“Searching for known blood cell markers on our ‘cell map’ we were able to identify the leukotriene biosynthesis pathway as a new regulator of early blood development” explains Professor Göttgens, one of the researchers involved in the study. “The availability of suitable blood donor cells is a limiting factor for many patients requiring bone marrow transplantation or specialized blood transfusions. The identification of new pathways driving normal blood cell development has the potential to improve our ability to generate blood cells for these patients in the laboratory”.
The ‘cell map’ data also offers new drug development opportunities. “If we know which genes are active at key developmental stages then we can develop drugs to target the important pathways and alter cell function in diseases such as congenital heart defects, one of the commonest diseases that require surgery in newborn babies” says lead author Dr Marioni. “We are making this comprehensive single cell map available to all researchers and hope it can be used to reveal previously unrecognised pathways contributing to mammalian development, whether that be lung, brain, liver or any other bodily tissue.”
Dr Andrew Chisholm, Head of Cellular and Developmental Sciences at Wellcome, said: "This is a comprehensive and deep characterisation of cell types present in the mouse embryo at this critical stage of development, creating a rich resource for the community. The study illustrates the power of single cell based techniques to understand the development of organs such as the heart and brain. It could also help researchers to improve methods for creating mature, specialised cells from stem cells.”
Dr Mariana Delfino-Machin, MRC Programme Manager for Cancer, said: “A major challenge in cell biology is piecing together the precise steps required to give a cell its identity. Until now, experimental methods have lacked the precision needed to perceive the differences between cells that explain how they develop different identities, and how these differences might cause disease. The characterisation of 20,000 individual cells in a post-implantation stage mouse embryo is an impressive achievement, which provides a comprehensive overview of the processes to guide cells to their individual identities at that stage.”
Professor Göttgens is part of the Wellcome – MRC Cambridge Stem Cell Institute and Dr John Marioni is jointly based at the Cancer Research UK Cambridge Institute, the European Bioinformatics Institute (EMBL-EBI) and the Wellcome Trust Sanger Institute. Both Professor Göttgens and Dr Marioni are partners in a wider initiative funded by Wellcome to apply single cell molecular analysis to decipher early mammalian development. This initiative builds on the internationally-leading expertise in stem cell and computational biology available across the Cambridge area, and also includes developmental biologist Professor Shankar Srinivas from the University of Oxford.
The support of the Wellcome, the Medical Research Council, Cancer Research UK, Bloodwise, the Leukemia and Lymphoma Society and the National Institutes of Health* has been invaluable in facilitating this research, and is gratefully acknowledged by the team.
Publication details:
Defining murine organogenesis at single cell resolution reveals a new pathway regulating blood progenitor formation. X. Ibarra-Soria, W. Jawaid, B. Pijuan-Sala, V. Ladopoulos, A. Scialdone, D. Jorg, R. Tyser, F. Calero-Nieto, C. Mulas, J. Nichols, L. Vallier, S. Srinivas, B. Simons, B. Göttgens and J. Marioni is published in Nature Cell Biology. DOI: 10.1038/s41556-017-0013-z

