Submitted by D. Birkett on Mon, 07/06/2021 - 12:21
In a new study published in the journal Nature, researchers have discovered how tumour cells can function to outcompete their neighbours and colonise the intestinal epithelium.
The study, funded by Cancer Research UK and Wellcome, showed that, by inducing local alterations in the surrounding tissue, mutant epithelial cells create an unfavourable environment, allowing tumour cells to gain a competitive advantage over their normal, non-mutant, neighbours.
As well as uncovering signatures for early cancer detection, this discovery paves the way for new kinds of prevention and intervention strategies that target the mechanisms that promote cellular crosstalk and cell competition.
Cancers evolve through a process of Darwinian selection, transitioning from a phase of neoplasia to invasive carcinoma and metastatic transformation.
This chain of events is preceded by a phase of “field cancerisation”, where cells bearing cancer “driver” mutations outcompete their normal neighbours. However, the mechanisms that confer a survival advantage on mutant cells have remained in question.
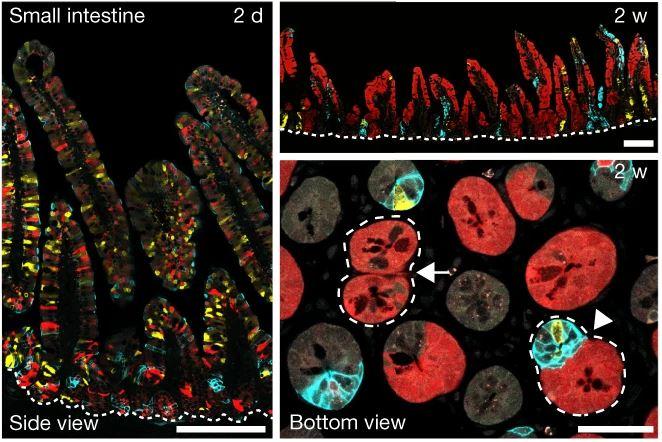
By designing a genetically modified mouse reporter system, researchers led by Benjamin Simons group leader at the Institute and Wellcome/CRUK Gurdon Institute and Dr Bon-Kyoung Koo at the Institute of Molecular Biotechnology (IMBA) in Vienna developed a novel method to label mutant and non-mutant cells in different colours simultaneously in the same tissue. The study was carried out in collaboration with researchers from across the Stem Cell Institute, including members of the Simons, Philpott and Lee groups.
By tracing the lineages of fluorescently labelled cells and their progenies –clones– in the glandular epithelium of the mouse intestine, they found that mutant epithelial cells can influence both the composition and fate behaviour of normal (wild-type) cells in neighbouring glands.
Lead author Min Kyu Yum said: “following oncogene activation, we found that mutant epithelial cells influence the fate of their wild-type neighbours both directly, through secretion of signalling factors, and indirectly through induced changes in the shared stromal tissue.”
“Through advances in single-cell gene expression profiling and organoid cultures, we were able to dissect the pathways that mediate cellular crosstalk”, said co-lead author Seungmin Han. “By targeting pre-malignant transformation, the Red2Onco system developed in this work promises new insights into a fundamental problem in cancer biology: why is it that, in some tissues and contexts, cells bearing oncogenic mutations are tolerated or even eliminated while, in others, they may transition to malignancy.”
Benjamin Simons commented that, “with further refinements of the ‘Red2Onco’ system, we are working with colleagues at IMBA and at the Cambridge Stem Cell Institute to investigate whether similar mechanisms of cellular crosstalk operate in other epithelial tissues including the stomach, lung and skin.”
This study, which was supported through a dedicated scheme to connect cancer biologists with researchers from the physical sciences, illustrates the power of multidisciplinary cooperation and innovation in advancing discovery science.
Read more about research in the Simons lab.

