Submitted by Laura Puhl on Thu, 03/02/2022 - 10:52
Researchers have identified a new therapeutic target that could be critical in developing improved treatments for thrombocythaemia
A new study from the Wellcome-MRC Cambridge Stem Cell Institute (CSCI), the Wellcome Sanger Institute, Department of Haematology at the University of Cambridge and Cambridge Institute for Medical Research (CIMR) investigates the process of platelet production, suggesting new perspectives on platelet regulation that could have potential therapeutic outcomes for treatment of essential thrombocythaemia (ET).
The paper, published recently in Blood looks for the first time into the role played by the protein coding gene CRLF3 in the mitigation of overproduction of platelets, and explores the possibility of applying the results to the creation of new therapies.
First author Dr Cavan Bennett said, “ET patients are still treated with decades old non-platelet specific therapies that have debilitating adverse effects. This collaborative research has identified a new player in platelet production and will allow further research into new targeted therapies for ET."
A target for platelet overproduction
Platelets are a component of blood developed in bone marrow, existing primarily to initiate clotting at sites of vessel injury. When these clots form in the wrong place, it causes strokes or heart attacks. Blood disorders like ET result in an overproduction of platelets, which puts individuals at risk of developing serious blood clots and other serious complications.
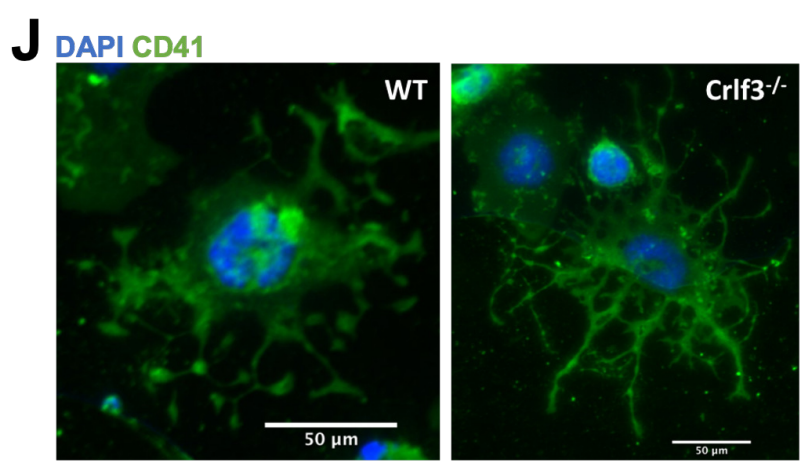
In existing research, it has been understood that platelets are produced in two stages. First, megakaryocytes (MKs) derived from haematopoietic stem cells (HSCs) form proplatelets (long branching protrusions). Second, the proplatelets release “beads-on-a-string” preplatelets into the blood stream that undergo fission to become mature platelets.
This research, conducted by Dr Bennett in the Ghevaert Lab (CSCI; Department of Haematology), distinguishes the preplatelet fission as a discrete third stage, critical for regulating the platelet count. Furthermore, the researchers found that CRLF3 can play a pivotal role in that regulation.
By studying mice with a deficiency of the CRLF3 gene, the team found that the presence or absence of this gene affects platelet production. When CLRF3 is not present, the preplatelets are hyper-stable, preventing them undergoing fission in the blood stream and allowing the spleen to filter them out of the system. Thus, in CRLF3-deficient mice, the platelets were correspondingly reduced as well.
Of this study, Dr Ghevaert says, “We identified a novel gene that appears to exert a very specific control on the platelet count. We show how influencing these gene can reduce the platelet count in the context of ET. This opens exciting avenues of research towards much more specific therapies for patients with ET."
New hope for therapies
Currently, most individuals with ET are treated with non-specific therapies over the long term to lower their platelet count. These existing treatments can cause anaemia, skin ulcers, bone marrow scarring, or a reduction of white blood cell count.
Though further research is still needed, this study’s results in targeting CRLF3 to reduce platelet counts is highly promising for future treatments for ET. In this study, the mice with deficient CRLF3 showed a sustained lower level of platelets while avoiding the adverse side effects of other treatments, namely bone marrow scarring and leukaemic transformation. With further study and increased understanding of CRLF3’s role in other cell types and relationships, there is potential for the development of a safer treatment for patients with ET.
“By systematic phenotypic screening of haematological parameters in mice we identified the Crlf3 gene. Remarkably, mice in which this gene had been deleted showed a persistently low platelet count, which we later showed was due to reduced platelet production. This suggests that treatments that block or inhibit CRLF3 could be used to treat patients with high platelet disorders.”
- Dr David Adams, Wellcome Sanger Institute

