Dr Jelle van den Ameele
Developmental neurobiology and mitochondrial genetics
Affiliation: Department of Clinical Neurosciences and MRC Mitochondrial Biology Unit
Biography
Jelle van den Ameele is a Wellcome Clinical Research Career Development Fellow at the Department of Clinical Neurosciences and MRC Mitochondrial Biology Unit. As a medical student at Ghent University (Belgium), he became interested in developmental neurobiology and joined Pierre Vanderhaeghen's lab in Brussels for his PhD, studying transcriptional regulation of brain development. His PhD and neurology training sparked an interest in mitochondrial genetics and metabolism. Thanks to postdoctoral funding from EMBO and Wellcome, he then joined the lab of Andrea Brand at the Gurdon Institute in Cambridge to study metabolism of brain development in Drosophila, and to develop novel approaches for genome-wide chromatin profiling in Drosophila and vertebrates.
As a positive culture champion at the School of Clinical Medicine, Jelle chairs the MBU WIDE committee, and coordinates the Mid-Career Fellows network at the School. As a neurologist, he continues to do clinical work and participates in the clinical research programme MitoCamb of the mitochondrial genetics clinic in Addenbrooke’s.
Research
The broad goal of our lab is to better understand tissue-specific presentation of mitochondrial and neurodegenerative diseases. We mainly study how mitochondrial dysfunction and mutations in the mitochondrial genome affect neural stem cell behaviour in Drosophila and mouse. The questions we address are:
- how mitochondrial dysfunction affects normal and pathological cell fate decisions in the developing brain,
- how transcription of the nuclear genome is regulated when a cell is confronted with mitochondrial dysfunction, and
- how mutations in the mitochondrial genome evolve over time, during brain development and aging.
Because we believe it is important to study these events in an in vivo context, in (stem) cells surrounded by their appropriate tissue environment, our primary model system is the fruit fly, Drosophila melanogaster. In addition, we actively seek to translate our findings and the technology we develop into mammalian model systems, in particular the mouse embryonic cortex.
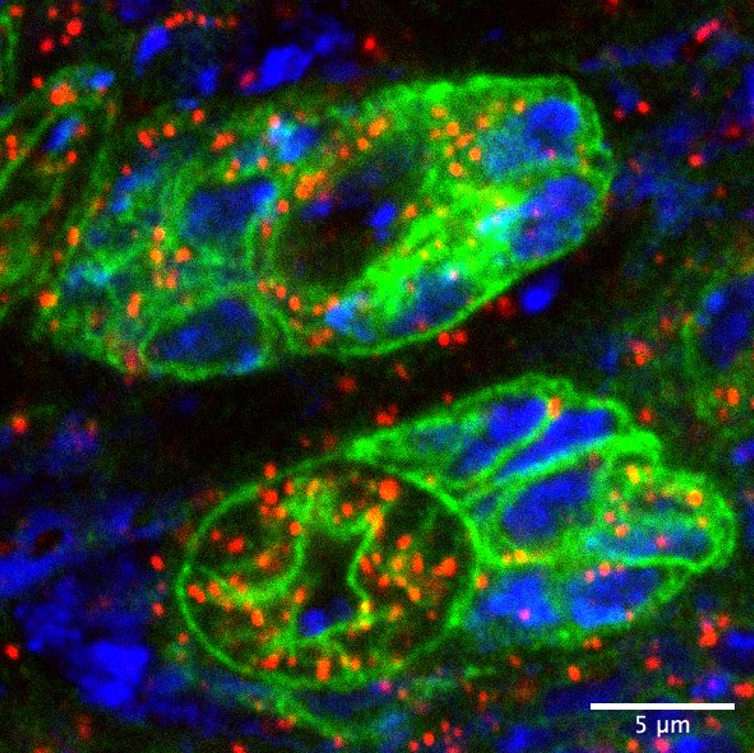
Neural stem cells and their progeny in the Drosophila brain (in green), with their mitochondrial genomes labelled in red and the nuclear genome in blue.
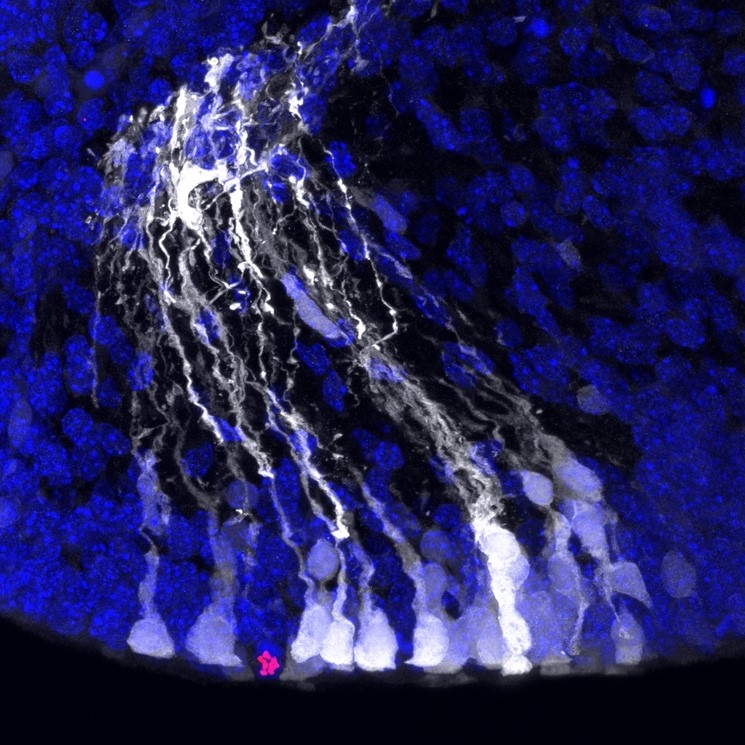
Neural stem cells (in white) in the mouse embryonic cortex, after ectopic activation of the Notch signalling pathway through in utero electroporation of the Notch intracellular domain. Nuclei in blue and dividing cells (PH3) in red.