Submitted by Laura Puhl on Wed, 23/02/2022 - 15:26
New research from the Wellcome-MRC Cambridge Stem Cell Institute (CSCI) and the Department of Physiology, Development, and Neuroscience at the University of Cambridge (PDN) explores the self-organisation of tissues in early embryos, offering an explanation for how cells sort themselves as they develop.
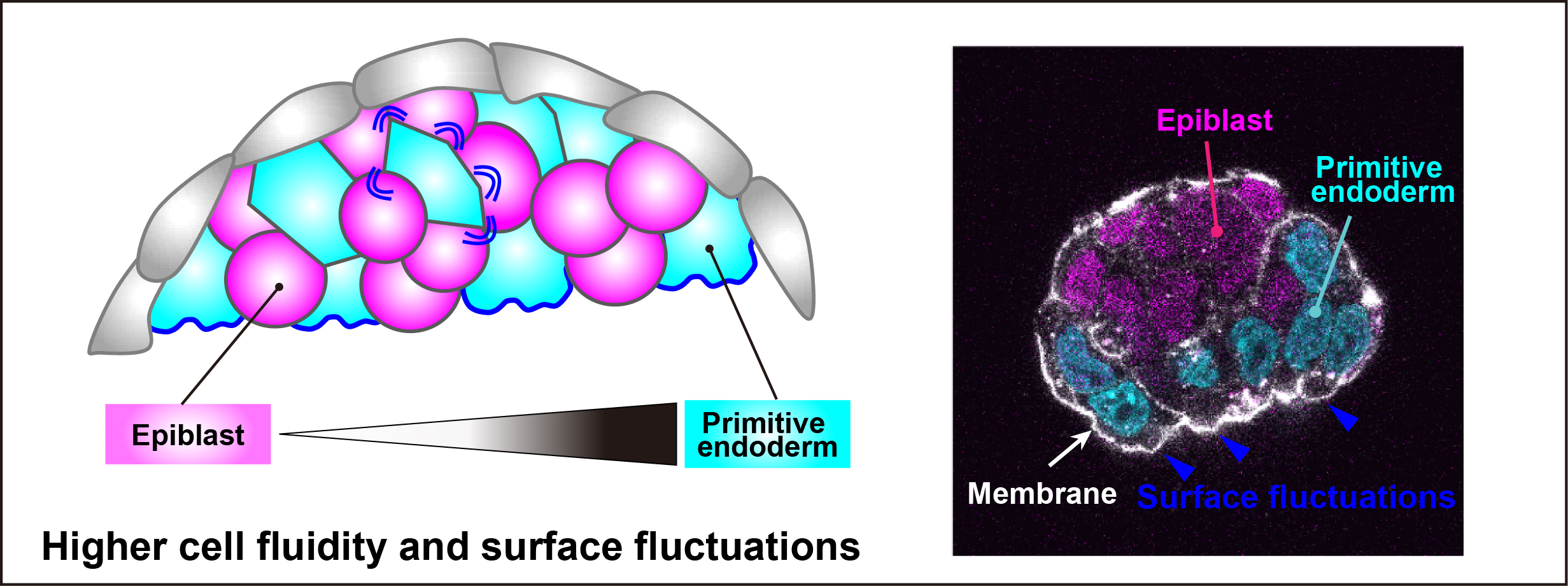
The paper, published 22 February in Cell, discovered that fluctuations on the surface of a cell ultimately determine whether it will become a foetus-forming cell, or become extraembryonic tissue responsible for managing patterning and nutrition of that foetus.
Lead author Dr Kevin Chalut comments, “To this point, no one had known how these two layers of the embryo segregated. We showed here that classical models of how cells separate from one another do not hold in this sorting process. Instead, the mechanism of sorting is that the primitive endoderm cells exhibit more surface fluctuations and become more fluid, helping them to sort to their proper location.”
Discovering new sorting mechanisms
In the early stages of mammalian development, an essential separation event occurs that is vital to the proper patterning of the embryo. After the blastocyst (the beginning stage of the embryo) is formed, a chemical signal initiates a reaction, causing the cells within to separate themselves into two distinct groups. One group (primitive endoderm) eventually becomes the yolk sac; the other (epiblast) later develops into the foetus. While the molecular requirements for this separation have been studied extensively, until now the physical mechanisms that support this separation have not been sufficiently investigated.
While classical sorting models explain cell sorting through static parameters such as cell surface tension or adhesive forces, they don’t consider the dynamics of these variables, and what those dynamics might mean in biological processes like embryonic development. Indeed, the more classical variables are unable to be used as a predictor for cell fate in the sorting of epiblast and primitive endoderm. This research, conducted by the team of scientists led by Dr Chalut, discovered that the overlooked dynamics of variables of such as surface tension in fact play a key role in how the cell will be sorted.
Through a combination of experiments, 3D modelling and time-lapse movies, the researchers were able to determine that although the early primitive endoderm and epiblast cells are physically similar in most ways, the primitive endoderm cells have more surface fluctuations and fluidity that helps get them where they need to go. Thus, rather than size and shape of a cell, it is actually surface fluctuations and cellular fluidity together that determine how the cell will be sorted.
Applications for future research
While this research explains cell sorting in the mouse embryo, the scientists next hope to apply this understanding of surface fluctuations to investigate how these dynamics influence other processes of cell organisation. In the future, this could even lead to better understanding of aberrant tissue formation. Dr Chalut says, “We believe this will not only help better understand early development, but it also provides insight for cell sorting in other important processes, not just in development but possibly in, for example, tumours.”