New charity - Myelopathy.org - launched
Submitted by Administrator on Fri, 17/05/2019 - 14:44
To raise awareness of myelopathy and to address gaps in our knowledge of the condition and how best to treat it, Cambridge Stem Cell Institute Affiliate, Dr Mark Kotter and colleague Ben Davies, together with Iwan Sadler, have launched Myelopathy.org, a charity that aims to give patients a voice and effect change.
Myelopathy is caused by arthritic changes affecting the spinal column of the neck. Because of the close proximity, these can exert pressure on the spinal cord and trigger a "slow motion spinal cord injury".
“If you haven’t heard of myelopathy, you are probably in good company,” said Dr Kotter. “Myelopathy is likely the most under-diagnosed neurological condition, yet it affects as many as a million adults in the UK.”
The onset of myelopathy is often subtle: symptoms include numb and clumsy hands, imbalance, and urinary problems. When left undiagnosed, it can progress with patients losing control of their hands and bladder, and becoming unable to walk. Myelopathy is now recognised as having one of the worst impacts on quality of life.
The actual number of patients who suffer from this condition remains unclear. Recent research by Dr Kotter's team analysed existing spinal MRI studies, with the results indicating that as many as one in 50 adults may be affected.
Read more here.
‘Mini-lungs’ reveal early stages of SARS-CoV-2 infection
Submitted by Administrator on Fri, 23/10/2020 - 17:29
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of scientists from the Cambridge Stem Cell Institute and collaborators in South Korea turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
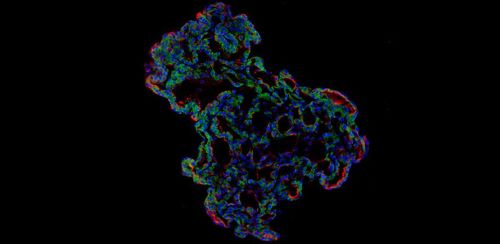
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organising alveolar-like 3D structures that mimic the behaviour of key lung tissue.
Dr Joo-Hyeon Lee, co-senior author, and a Group Leader at the Wellcome-MRC Cambridge Stem Cell Institute, University of Cambridge, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in South Korea who was diagnosed with COVID-19 on 26 January 26 2020 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Dr Young Seok Ju, co-senior author, and an Associate Professor at Korea Advanced Institute of Science and Technology. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr Lee.
Reference
Jeonghwan Youk et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell; 21 Oct 2020; DOI: 10.1016/j.stem.2020.10.004
Funding
The research was supported by: the National Research Foundation of Korea; Research of Korea Centers for Disease Control and Prevention; Ministry of Science and ICT of Korea; Ministry of Health & Welfare, Republic of Korea; Seoul National University College of Medicine Research Foundation; European Research Council; Wellcome; the Royal Society; Biotechnology and Biological Sciences Research; Suh Kyungbae Foundation; and the Human Frontier Science Program.
Image caption
Representative image of three-dimensional human lung alveolar organoid showing alveolar stem cell marker, HTII-280 (red) and SARS-CoV-2 entry protein, ACE2 (green). (Credit: Jeonghwan Youk, Taewoo Kim, and Seon Pyo Hong)
Live cells discovered in human breast milk could aid breast cancer research

Submitted by Laura Puhl on Thu, 27/01/2022 - 16:42
Unlocking the secrets of human milk cells
A new study from the Wellcome-MRC Cambridge Stem Cell Institute (CSCI) and the Department of Pharmacology at the University of Cambridge explores the cellular changes that occur in human mammary tissue in lactating and non-lactating women, offering insight into the relationship between pregnancy, lactation, and breast cancer.
Breast tissue is dynamic, changing over time during puberty, pregnancy, breastfeeding, and aging. The paper, published today (28th January) in Nature Communications, focuses on the changes that take place during lactation by investigating cells found in human milk.
This research, led by Dr Alecia-Jane Twigger of CSCI, led to the discovery that the cells in milk, once thought to be dead or dying, are in fact very much alive. These living cells provide researchers with the chance to study not only the changes that occur in mammary tissues during lactation, but also insight into a potential early indicator of future breast cancer development.
The researchers collected voluntary breastmilk samples from lactating women, as well as samples of non-lactating breast tissue donated from women who elected to have aesthetic breast reduction surgery. Using single-cell RNA sequencing analysis, the team conducted a novel comparison of the composition of the mammary cells taken using these two methods, identifying the distinctions between lactating and non-lactating human mammary glands.
While accessing breast tissue for study relies on donors already undergoing surgery, breast milk samples are much simpler to acquire. Breast milk donors are engaged via midwives or women’s networks (an undertaking made more challenging by the pandemic) and agree to share their samples over time. Typical daily production for lactating women is between 750-800ml per day, and the sample size for Twigger’s research is on average a mere 50ml, an amount which can contain hundreds of thousands of cells for study.
By collecting these samples donated from breastfeeding women – samples now known to contain living and viable cells – researchers have the opportunity to capture dynamic cells in a non-invasive way. This greater ease of access to breast cells can open the door to more studies on women’s health going forward.
Dr Twigger is a Research Associate in Dr Khaled’s laboratory (CSCI; Pharmacology Department at University of Cambridge). This paper and its findings are part of the Human Breast Cell Atlas project funded by the MRC.
“I believe that by studying human milk cells, we will be able to answer some of the most fundamental questions around mammary gland function such as: how is milk produced? Why do some women struggle to make milk? and what strategies can be employed to improve breastfeeding outcomes for women?”
- Dr Alecia-Jane Twigger, CSCI
“The first time Alecia told me that she found live cells in milk I was surprised and excited about the possibilities. We hope this finding will enable future studies into the early steps of breast cancer.”
- Dr Walid Khaled, CSCI, Department of Pharmacology
Stem Cells IRC workshop winners announced

Submitted by Laura Puhl on Fri, 18/11/2022 - 18:11
Winners from the Collaboration Creation Days workshop have been awarded this week for their proposals presented at the end of the event.
In September, the Wellcome-MRC Cambridge Stem Cell Institute Interdisciplinary Research Centre, together with Sensor CDT, Cambridge Centre for Physical Biology, and the Department of Chemical Engineering and Biotechnology, hosted an interdisciplinary workshop for Cambridge academics.
‘Collaboration Creation Days: Where cell biology, physics and sensors meet’ was a first-time Stem Cells IRC workshop designed to unite scientists from various fields and career stages to test their ideas, build an interdisciplinary team, and pitch their research to an expert funding panel for a chance to win funding for their project.

Throughout the 3.5 day workshop, the participants (PhD students, PostDocs and early career scientists) took part in lectures and group work before presenting their own research ideas.
In the first two days of the workshops the participants heard from world-class scientists about their interdisciplinary research, associated challenges and opportunities. Speakers were Prof. George Malliaras, Dr. Anthie Moysidou, Prof. Kevin Chalut, Dr. Matthias Zilbauer, Dr. Alex Justin and Prof. Madeline Lancaster.
On the last day the students presented their research pitch to an expert panel including Prof. Roisin Owens, Prof. Serena Best, Dr. Maria Alcolea and Dr. Mekayla Storer and competed to win a research grant to work on the project.
The groups are being awarded up to £4,000 to continue work on their proposed projects. An update to those projects will be shared here on completion.
The winning groups are:
Group 1: Nino, Shirom and Ziqi with the project proposal "Deciphering the Spatial Determinants of Human Lung Development"
Group 2: Sabila, Udit, Kyriacos, Chi Ki with the project proposal " Proposal to investigate the role of adaptor proteins in mRNA transport in the oocyte-to-embryo transition in Drosophila"
Group 3: Meng, Ana, Jan and Aish with the project proposal "Observing oxidative stress and morphogenesis alterations during mechanical force regulation in chicken embryo stem cells"
This workshop will be repeated again in future years for researchers who are interested in multidisciplinary work.




